Articles
- Page Path
- HOME > Kosin Med J > Volume 39(1); 2024 > Article
-
Case report
Primary gastric leiomyosarcoma: a case report and literature review -
Yedaun Lee

-
Kosin Medical Journal 2024;39(1):60-65.
DOI: https://doi.org/10.7180/kmj.23.118
Published online: August 18, 2023
Department of Radiology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- Corresponding Author: Yedaun Lee, MD, PhD Department of Radiology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, 875 Haeun-daero, Haeundae-gu, Busan 48108, Korea Tel: +82-51-797-0381 Fax: +82-51-797-0379 E-mail: chosai81@gmail.com
• Received: April 10, 2023 • Revised: June 20, 2023 • Accepted: July 5, 2023
© 2024 Kosin University College of Medicine.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,494 Views
- 21 Download
Abstract
- After separating gastrointestinal (GI) stromal tumors from true smooth muscle tumors of the GI tract, leiomyosarcoma (LMS) of the GI tract has become a rare tumor. Gastric LMS is extremely rare and accounts for 0.1% of all cases of LMS in the GI tract. There are few English-language reports of gastric LMS describing radiologic findings. Here, we report a case of gastric LMS and review the recent literature focusing on radiologic findings. An 80-year-old female patient was referred for evaluation of a gastric mass accompanied by severe anemia. The physical examination revealed no specific findings except for an anemic conjunctiva. Laboratory data showed a low hemoglobin level of 5.1 g/dL. Endoscopy revealed a huge subepithelial mass in the posterior wall of the gastric body. Contrast-enhanced computed tomographic images showed an intraluminal protruding enhancing mass with an internal stalk appearance in the gastric body. There was no internal necrosis or calcification. The patient underwent subtotal gastrectomy and was diagnosed with primary gastric LMS. The diagnosis of gastric LMS is challenging due to its rarity. Our case report suggests that the presence of an internal stalk or spouting appearance can help prompt the radiologist to consider gastric LMS in the differential diagnosis.
- After the separation of gastrointestinal stromal tumors (GIST) from true smooth muscle tumors of the gastrointestinal (GI) tract [1], leiomyosarcoma (LMS) of the GI tract has become a rare tumor [2,3]. Gastric LMS is extremely rare and accounts for 1% of all gastric tumors [4]. Of all LMS in the GI tract, only 0.1% of these tumors occurred in the stomach [5]. There are fewer than 20 case reports of gastric LMS written in English [2-4,6-20]. However, these reports focused on histologic findings, and only a few described radiologic findings of gastric LMS [10,12,14-17]. Here, we report a case of gastric LMS and review the recent literature focusing on radiologic findings.
Introduction
- Ethical statements: This study was exempted from review by the Institutional Review Board (IRB) of Haeundae Paik Hospital (IRB No. HPIRB 2023-01-011). The requirement for informed consent was waived by the IRB.
- The 80-year-old woman was referred to our emergency department for further evaluation of a gastric mass with severe anemia. She underwent an endoscopy 6 months ago and became aware of the presence of a gastric tumor. The endoscopic impression indicated GIST. However, she did not pursue treatment at that time. She had a history of hypertension, for which she has been treated for 5 years. Physical examination revealed no specific findings except for an anemic conjunctiva. The patient’s vital signs were blood pressure: 90/50 mmHg, respiratory rate: 20 respirations/min, heart rate: 84 beats/min, and temperature: 36.5 °C. Laboratory data showed a low hemoglobin level of 5.1 g/dL. After adequate fluid resuscitation and blood transfusion, the esophagogastroscopy examination was performed. Endoscopy revealed a huge mass in the posterior wall of the gastric body with mucosal erosion and ulceration (Fig. 1). Endoscopic biopsy was performed, and the results revealed a malignant spindle cell neoplasm. Multidetector computed tomography (CT) revealed a 10.5 cm-sized intraluminal protruding mass with the lobulated contour in the posterior gastric body (Fig 2). On the pre-contrast image, there is no calcification or hemorrhage. The mass shows gradual enhancement and internal stalk. Also, the mass does not show internal necrosis. There is no regional or distant lymphadenopathy. On positron emission tomography-CT, uptake of fluoro-2-deoxy-D-glucose was noted in the stomach, and there is no distant metastasis. A subtotal gastrectomy was performed. Gross findings revealed a yellowish gray ulcerofungating soft tissue mass measuring 11.0×8.5 cm (Fig. 3). The mass shows an internal stalk on the gross image. Microscopically, the mass was composed of malignant spindle cells without necrosis. Mitotic count was 290/10 high power fields (Fig. 4A). Immunohistochemical stain was positive for smooth muscle actin and desmin but negative for c-kit, DOG-1, S100, and CD34 (Fig. 4B). Forty regional lymph nodes were dissected, and the pathologic findings demonstrate no evidence of metastasis. The tumor was diagnosed with a primary gastric LMS. On follow-up abdominopelvic CT 7 years after surgery, the patient had no disease recurrence (Fig. 5).
Case
- Gastric LMS is extremely rare in post-GIST era, and there have been only 17 case reports in English literature since 2003 (Table 1). The mean age of gastric LMS was 51.2 years (range, 16–74 years), with occurred predominantly in the gastric body (10/17 cases). The most common symptom was bleeding, causing hematemesis, anemia, or melena. The mean size of the tumor was 7.3 cm (range, 1–22 cm). The symptom can vary depending on the tumor's location and size. The gross appearance of the tumor varied from the polypoid, exophytic, fungating, and ulcerating mass. Distant metastasis at diagnosis is not common (2/17 cases). The common metastatic site is the liver and lung. Due to the rarity of gastric LMS, there is no consensus on its treatment. However, complete resection with negative margins is generally regarded as the most effective approach. In cases where the tumor is confined to the submucosa and surgery is challenging, endoscopic resection can be a viable option. The effectiveness of chemotherapy and radiotherapy in treating gastric LMS remains uncertain [18].
- CT scan is performed primarily in patients with gastric LMS to make a diagnosis and evaluate the presence of metastasis. However, at initial diagnosis, it is challenging to differentiate gastric LMS from other subepithelial gastric tumors because it is very rare, and radiologic findings of gastric LMS have not been sufficiently reported to date. The protruding enhancing mass is the most common finding of gastric LMS, according to a few reports reporting radiologic findings [9,10,14-17]. Among these reports, three cases showed protruding mass es with internal stalk-like appearance [10,16,17], and our case also showed a similar appearance in CT scans and gross pathology (Figs. 2, 3). Masuzawa et al. [10] showed an in vitro magnetic resonance imaging correlating to histopathologic findings. They described “spouting sign,” which indicates a heterogenous mass having high-intensity areas separated by isointense radial septum-like structures. The corresponding pathologic exam revealed this was composed of septum-like thin fibrovascular stroma alternating with myxedematous areas in the tumor. Four cases of seven radiologically described gastric LMS, including our case, were presented as protruding masses with internal stalks. Although these findings cannot be generalized that it appears in all gastric LMS, if the gastric subepithelial mass showed an internal stalk or spouting appearance, it could help radiologists consider gastric LMS in their list of differential diagnoses. Further investigation with more cases is needed.
- In conclusion, we report a rare case of gastric LMS and review radiologic findings. Although there are no established radiologic findings, a mass with an internal stalk or spouting appearance can be helpful for the radiologist to consider the gastric LMS as their differential diagnosis.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
All work was performed by YL.
Article information
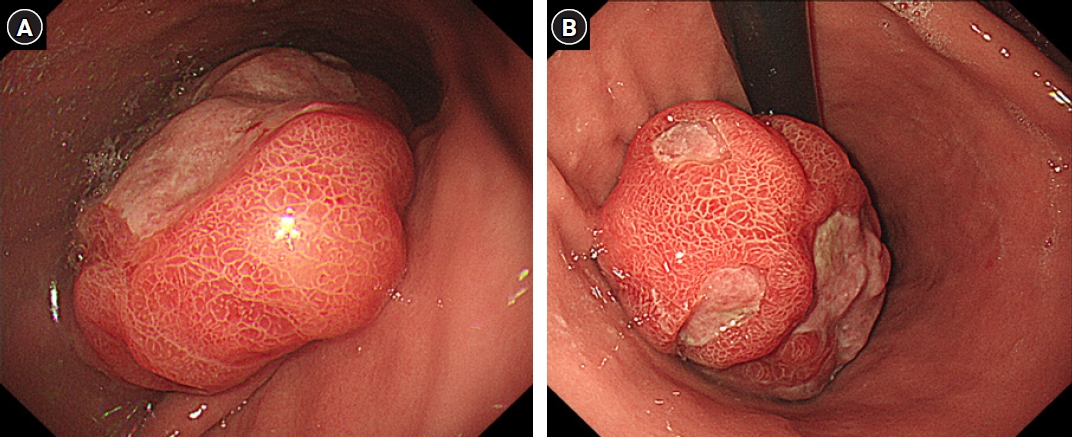
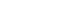
Fig. 1.Gastroduodenoscopy demonstrates a huge intraluminal mass with erosion and ulcers, measuring approximately 11 cm in diameter, in the posterior wall of the gastric body (A, B). Mucosal gastric folds surrounding the lesion are present.
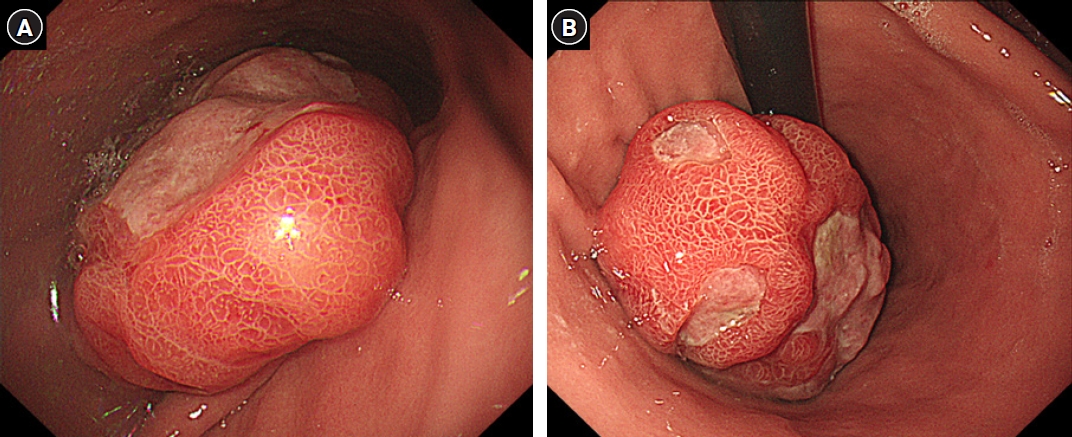

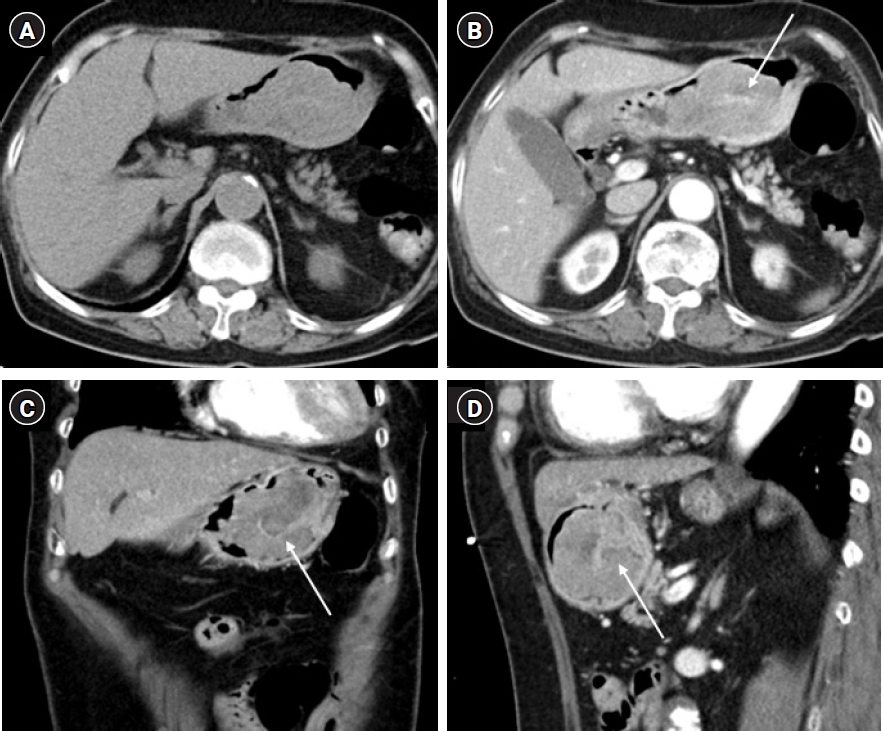
Fig. 2.Axial pre-contrast (A) and contrast-enhanced (B) computed tomography scans show an intraluminal, protruding, lobulating, enhancing mass arising from the posterior wall of the gastric body. On the pre-contrast scan (A), there is no visible calcification or necrotic component. An internal stalk-like appearance (arrows) is noted on enhanced axial (B), coronal (C), and sagittal (D) images.


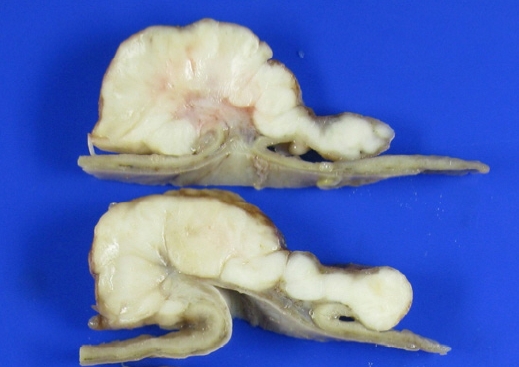
Fig. 3.A gross examination of the stomach revealed a yellowish gray ulcerofungating mass measuring 11.0×8.5 cm. The cut surface of the mass showed an internal stalk.
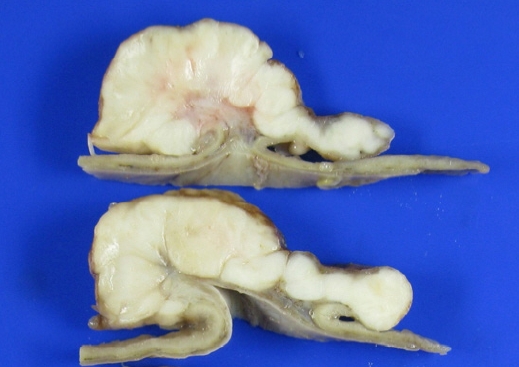

Fig. 4.(A) Photomicrography shows proliferation of spindle cells with high cellularity and mitotic count (hematoxylin and eosin stain, ×200). (B) Immunohistochemical staining for desmin and smooth muscle actin shows diffuse and strong positivity (×200). The tumor cells are negative for c-kit, CD34, DOG-1, and S-100.
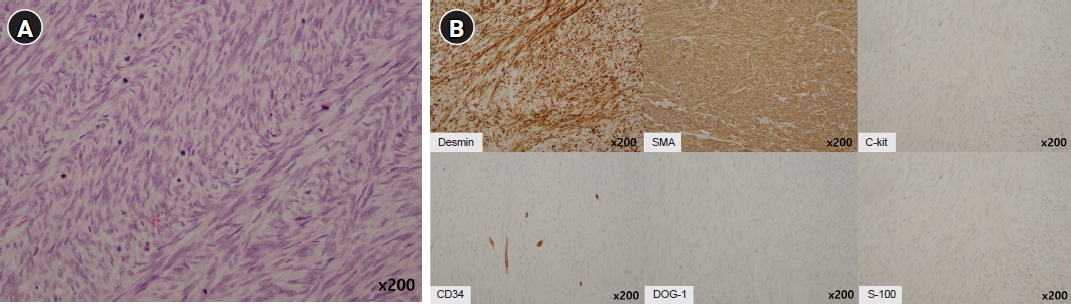

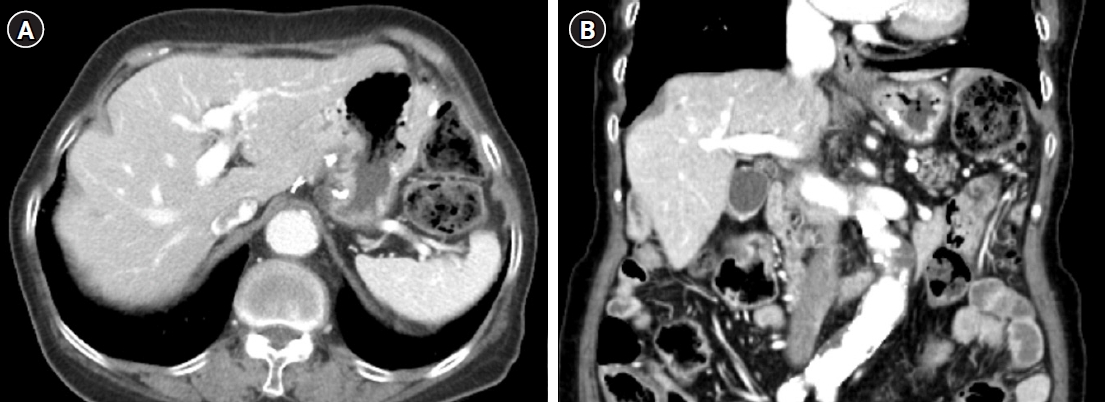
Fig. 5.Follow-up axial (A) and coronal (B) abdominopelvic computed tomography taken 7 years after surgery shows no evidence of recurrence or metastasis.
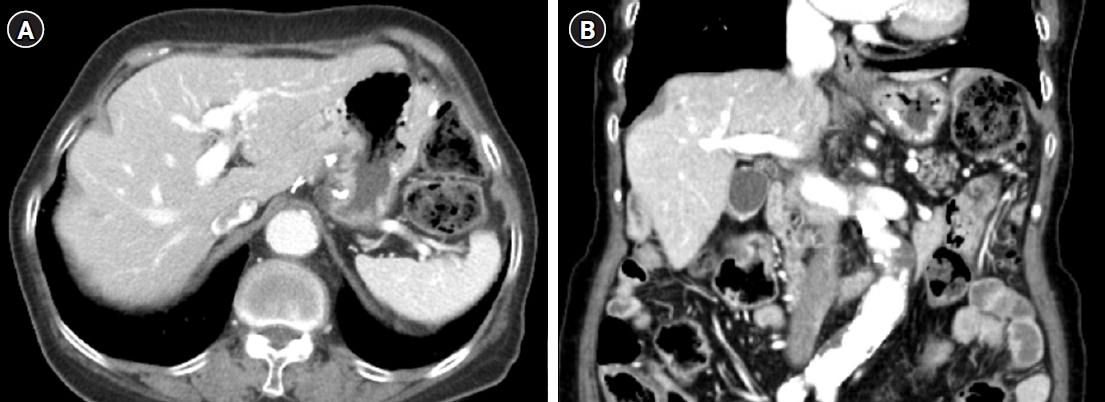

Table 1.Summary of cases with gastric leiomyosarcoma
| Author (year) | Age(yr)/sex | Symptoms | Site | Size (cm) | Gross appearance | Metastasis at diagnosis | Mitoses | Outcome |
|---|---|---|---|---|---|---|---|---|
| Ayoola et al. (2003) [6] | 68/M | Melena/anemia | Body, antrum | NR | Nodular | Liver metastasis | NR | Death (3 mo) |
| Insabato et al. (2004) [7] | 65/M | NR | NR | 8.5 | Fungating | None | NR | Death (24 mo) |
| Biswas et al. (2006) [4] | 28/F | Hematemesis | Body | 6 | Ulcerated polypoid | None | >20/50 HPF | NR |
| Agaimy and Wunsch (2007) [2] | 72/F | NR | Body | 2.5 | Ulcerated polypoid | None | 5/10 HPF | NR |
| Pauser and Grimm (2007) [8] | 37/M | Abdominal pain | Antrum | 1 | Polypoid | None | 20/50 HPF | No recur (36 mo) |
| Soufi et al. (2009) [9] | 16/F | Hematemesis | Body | NR | Exophytic | None | NR | No recur (18 mo) |
| Masuzawa et al. (2009) [10] | 29/F | Anemia | Body | 11 | Polypoid | None | 100/10 HPF | No recur (8 mo) |
| Aggarwal et al. (2012) [11] | 26/M | Melena | Fundus | 7.2 | Exophytic | None | >20/10 HPF | Follow-up loss |
| Yamamoto et al. (2013) [3] | 51/M | NR | NR | 2.5 | Ulcerated polypoid | None | 40-50/10 HPF | No recur (18 mo) |
| Hilal et al. (2016) [13] | 74/M | Weight loss | NR | 8 | Lobulated | None | 2/50 HPF | No recur (9 yr) |
| Mehta et al. (2018) [14] | 47/M | Abdominal pain | Body | 13 | Exophytic | None | NR | Liver metastasis (3 yr) |
| Sato et al. (2018) [15] | 74/F | Screening | Body | 1.5 | Ulcerated polypoid | None | 18/10 HPF | No recur (3 yr) |
| Hasnaoui et al. (2018) [16] | 63/F | Bleeding | Cardia | 7 | Protruding | None | NR | NR |
| Kang et al. (2019) [17] | 57/F | Melena | Body | 13 | Polypoid | None | NR | Death (1 yr) |
| Garg et al. (2020) [18] | 54/M | Anemia | Body | 3 | Ulcerating | None | 10/10 HPF | No recur (1 yr) |
| Gubatan and Shah (2020) [19] | 50/M | Bleeding | Body | 3.5 | Ulcerated polypoid | None | 12/10 HPF | Not treated |
| Al-Yousofy et al. (2020) [20] | 60/F | Abdominal pain | NR | 22 | NR | Liver, lung, LN metastases | NR | No recur (9 mo) |
- 1. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577–80.ArticlePubMed
- 2. Agaimy A, Wunsch PH. True smooth muscle neoplasms of the gastrointestinal tract: morphological spectrum and classification in a series of 85 cases from a single institute. Langenbecks Arch Surg 2007;392:75–81.ArticlePubMedPDF
- 3. Yamamoto H, Handa M, Tobo T, Setsu N, Fujita K, Oshiro Y, et al. Clinicopathological features of primary leiomyosarcoma of the gastrointestinal tract following recognition of gastrointestinal stromal tumours. Histopathology 2013;63:194–207.ArticlePubMed
- 4. Biswas M, Rahi R, Tiwary SK, Khanna AK, Khanna R. Leiomyosarcoma of stomach: a case report. Kathmandu Univ Med J (KUMJ) 2006;4:510–2.PubMed
- 5. Miettinen M, Fetsch JF. Evaluation of biological potential of smooth muscle tumours. Histopathology 2006;48:97–105.ArticlePubMed
- 6. Ayoola EA, Arab MM, Tadros NM, Ahmed MA, Banzal SS, Abbo HM, et al. Multicentric leiomyosarcoma of the stomach. Saudi J Gastroenterol 2003;9:79–81.PubMed
- 7. Insabato L, Di Vizio D, Ciancia G, Pettinato G, Tornillo L, Terracciano L. Malignant gastrointestinal leiomyosarcoma and gastrointestinal stromal tumor with prominent osteoclast-like giant cells. Arch Pathol Lab Med 2004;128:440–3.ArticlePubMedPDF
- 8. Pauser U, Grimm H. Intramucosal leiomyosarcoma of the stomach following hereditary retinoblastoma in childhood: a case report and review of the literature. World J Surg Oncol 2008;6:131.ArticlePubMedPMCPDF
- 9. Soufi M, Errougani A, Chekkof RM. Primary gastric leiomyosarcoma in young revealed by a massive hematemesis. J Gastrointest Cancer 2009;40:69–72.ArticlePubMedPDF
- 10. Masuzawa N, Kishimoto M, Nishimura A, Ichiba N, Aoki E, Yanagibashi K, et al. Gastric leiomyosarcoma manifesting peculiar findings: radiological-pathological correlation. Pathol Int 2009;59:306–11.ArticlePubMed
- 11. Aggarwal G, Sharma S, Zheng M, Reid MD, Crosby JH, Chamberlain SM, et al. Primary leiomyosarcomas of the gastrointestinal tract in the post-gastrointestinal stromal tumor era. Ann Diagn Pathol 2012;16:532–40.ArticlePubMed
- 12. Rou WS, Ju JS, Kang SH, Moon HS, Sung JK, Lee BS, et al. A case of gastric leiomyosarcoma with multiple metastases. Korean J Gastroenterol 2015;65:112–7.ArticlePubMed
- 13. Hilal L, Barada K, Mukherji D, Temraz S, Shamseddine A. Gastrointestinal (GI) leiomyosarcoma (LMS) case series and review on diagnosis, management, and prognosis. Med Oncol 2016;33:20.ArticlePubMedPDF
- 14. Mehta V, Rajawat M, Rastogi S, Phulware RH, Mezencev R. Leiomyosarcoma of the stomach with metastasis to the liver: a case report with review of the literature. Future Sci OA 2017;4:FSO264.ArticlePubMedPMC
- 15. Sato T, Akahoshi K, Tomoeda N, Kinoshita N, Kubokawa M, Yodoe K, et al. Leiomyosarcoma of the stomach treated by endoscopic submucosal dissection. Clin J Gastroenterol 2018;11:291–6.ArticlePubMedPDF
- 16. Hasnaoui A, Jouini R, Haddad D, Zaafouri H, Bouhafa A, Ben Maamer A, et al. Gastric leiomyosarcoma and diagnostic pitfalls: a case report. BMC Surg 2018;18:62.ArticlePubMedPMCPDF
- 17. Kang WZ, Xue LY, Tian YT. Leiomyosarcoma of the stomach: a case report. World J Clin Cases 2019;7:3575–82.ArticlePubMedPMC
- 18. Garg R, AlRajjal A, Berri R, Barawi M. Primary gastric leiomyosarcoma: a case report and review of the literature. J Gastrointest Cancer 2020;51:335–40.ArticlePubMedPDF
- 19. Gubatan J, Shah N. Gastric leiomyosarcoma unmasked by bleeding from a percutaneous endoscopic gastrostomy tube. ACG Case Rep J 2020;7:e00301.ArticlePubMedPMC
- 20. Al-Yousofy F, Alshargabi G, Ahmed F, Almohtadi A, Fazea M, Altam A. Primary huge gastric leiomyosarcoma with multiple metastases in a 60-year-old female: a case report. Pan Afr Med J 2022;42:223.ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 


 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite