Articles
- Page Path
- HOME > Kosin Med J > Volume 33(3); 2018 > Article
-
Original Article
Analysis of factors related systemic recurrence after breast conserving surgery in stage I breast cancer - Yoon-Seok Kim, Dong-Won Ryu, Chung-Han Lee
-
Kosin Medical Journal 2018;33(3):289-296.
DOI: https://doi.org/10.7180/kmj.2018.33.3.289
Published online: December 31, 2018
Department of surgery, College of Medicine, Kosin University, Busan, Korea.
- Corresponding Author: Chung Han Lee, Department of Surgery, College of Medicine, Kosin University, 262, Gamcheon-ro, Seo-gu, Busan, 49267, Korea. Tel: +82-51-990-6462, Fax: +82-51-246-6093, mammomaster@naver.com
• Received: December 27, 2013 • Revised: January 9, 2014 • Accepted: January 13, 2014
Copyright © 2018 Kosin University College of Medicine
Articles published in Kosin Medical Journal are open-access, distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 938 Views
- 3 Download
Abstract
-
Objectives
- In these days, patients with stage I breast cancer have increased by regular health examination and diagnostic tool development. The aim of this retrospective study is to identify systemic recurrence related factors after breast conserving surgery (BCS) for stage I breast cancer.
-
Methods
- In this study, we analyzed the correlation between systemic recurrence and pathologic factors. We reviewed 223 patients who underwent BCS for stage I breast cancer. Postoperative pathologic factors, recurrent rates and sites were studied. In addition, preoperative patients'data were also collected. Statistical analysis was done by using PASW 16.0 (SPSS Inc., Chicago, IL, USA).
-
Results
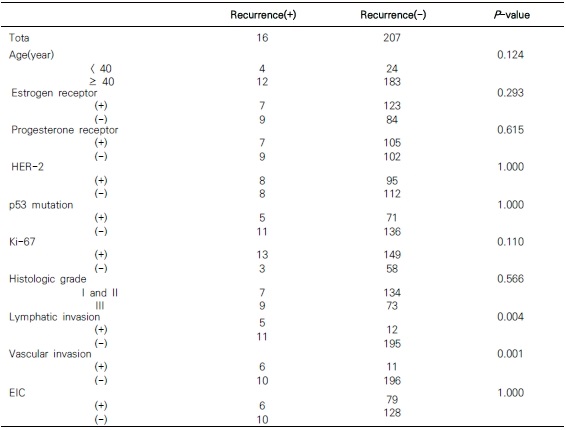
- Systemic recurrence was found in 16 patients (7.17%) within 5 years after primary surgery. 5 patients had lymphatic invasion and 6 patients had vascular invasion. Lymphatic and vascular invasion had statistical correlation with systemic recurrence (P = 0.004, P = 0.001).
-
Conclusions
- In this retrospective study, we can conclude that vascular invasion and lymphatic invasion are related systemic recurrence after BCS for stage I patients. Further studies with large cohort will be required to fully understand the risk factors of systemic recurrence for stage I breast cancer patients.
- Subjects
- The medical records of patients that received breast conserving surgery for stage I breast cancer from January 2002 to December 2007 were retrospectively analyzed, and the last tracking of the patients was November 2012. Subjects consisted of patients who had a record of at least 5 years of tracking after surgery, and the recurrence rates and parts of recurrence within 5 years of surgery were analyzed. Those who received surgery or chemotherapy due to primary cancers that occurred in organs other than breasts, and those who received neoadjuvant chemotherapy or radiotherapy to lower the stages of breast cancer were excluded. A total of 223 patients were examined as the subjects for this study, and core needle biopsy was used for pre-operative diagnosis. The marginal zones of clinical specimens and sentinel lymph nodes were checked through the frozen section test during surgery. The marginal zones of all clinical specimens and sentinel lymph nodes turned out to have ‘no tumor,’ which was confirmed through the final post-operative pathological examination. All tumors were found to be 2cm or smaller through the pathological examination. Chemotherapy was omitted for patients aged 35 or above who satisfied the requirements of histological grade 1 and positive hormone receptor, while all other patients received chemotherapy.
- Pathological factors of patients were analyzed through ER, PR, HER-2 protein, p53 mutations, Ki-67, histological grade, lymphovascular invasion, blood vessel invasion and EIC analysis with post-operative pathological examination, and pre-operative data of patients were also examined.
- Post-operative pathological factors were analyzed through the immunohistochemical (IHC) test, and ER and PR are considered positive when at least 10% is expressed on the IHC test, and HER-2 protein expression was exhibited when it is 3+ on the IHC test or is positive on FISH (fluorescence in situ hybridization). EIC is considered positive when at least 25% is expressed, Ki-67 is exhibited when at least 14% is expressed, and p53 mutation occurred when at least 1% is expressed.
- Statistical analysis
- A chi-squared test through PASW 16.0 (SPSS Inc., Chicago, IL, USA) was used to conduct multivariate analysis, and the results were considered statistically significant if P-value was below 0.05.
MATERIALS AND METHODS
- Histological characteristics of all patients
- Table 1 shows the clinical and pathological characteristics of all patients. All patients were female, and 28 were below age 40 (12.6%) and 195 were age 40 and above (87.4%). 130 patients (58.3%) exhibited ER expression, and 112 (50.2%) exhibited PR expression, while 103 patients (46.2%) exhibited HER-2 protein expression. For histological grade, 141 patients (63.2%) were in Grade I/II, and 82 patients (36.8%) in Grade III. 17 patients (7.6%) exhibited lymphovascular and blood vessel invasion respectively, and 76 patients (34.1%) exhibited p53 mutations. Ki-67 was expressed in 162 patients (72.6%), and EIC was positive in 85 patients (38.1%).
- Statistical analysis of post-operative pathological factors and systematic recurrence
- Table 2 shows the correlation between post-operative pathological factors and systematic recurrence. 4 patients below age 40 and 12 patients age 40 and above showed recurrences, but there was no statistical significance according to age (P = 0.124). Moreover, 7 of the patients that showed recurrences also showed ER expression, which had no statistical significance (P = 0.293), and the same was true for PR (P = 0.615). There were 8 patients who showed HER-2 protein expression, which was not related to recurrence (P = 0.799), and p53 mutations and Ki-67 expression were also not related (P = 1.000, P = 0.110). Histological grade and EIC also did not indicate any relevance to systematic recurrence (P = 0.566, P = 1.000). Of all the patients that showed recurrences, 5 exhibited lymphovascular invasion and 6 exhibited blood vessel invasion, which were related to systematic recurrence (P = 0.004, P = 0.001).
- Analysis on parts of recurrence and recurrence rates
- Table 3 shows parts of post-operative recurrence and recurrence rates. The average observation period of all patients was 82.3 months. Systematic recurrence was found in 16 patients (7.17%), with the liver was the organ were recurrences most frequently occurred (5 patients). Of the remaining patients with recurrences, 4 showed recurrences in bones and 2 in the brain. There was also one case each for the contralateral breast, contralateral breast and lung, lung and liver, brain and liver, and bone and brain simultaneously.
RESULTS
- Surgical treatment of breast cancer began when William Halsted performed radical mastectomy in 1894. Due to recent changes in the basic concept of breast cancer, efforts are being made to minimize the extent of surgery including the breast conserving surgery procedure. In 1990, breast conserving surgery was acknowledged to reveal “no difference in terms of survival rates compared to mastectomy for patients with stages 1 and 2.” In addition, with its associated aesthetic and psychological benefits, breast conserving surgery is positioning itself as a universal treatment for breast cancer.45678
- With the recent development of health examination and diagnosis technology, the number of stage I breast cancer patients is increasing. The rate of early breast cancer in Korea was 23.8% in 1996, but it increased to 24.5% in 2004. Breast cancer patients may show a favorable prognosis if there is no axillary lymph node metastasis, but there are recurrences in 20–30% of all breast cancer patients during the survival period.89
- Well-known prognostic factors related to breast cancer include the state of surgical resection margin, EIC, patient age, tumor size, lymph node metastasis, HR expression, histological grade, tumor marker, DNA proliferation marker (Ki-67, S demarcation, mitotic index), lymphovascular and blood vessel invasion.10111213
- Many studies have revealed that patient age below 35 or 40 is a risk factor for recurrence.568111314151617181920 Kim et al. stated that the difference in recurrence rates among age groups originates from the tumor-biological difference. However, the present study did not reveal any relevance between age and systematic recurrence.13 Whether HR is expressed or not is also known to be a factor related to prognosis of patients.202122 However, a few studies have claimed that the state of HR has no relation to local or systematic recurrence, and this study also corroborated this.814 In general, overexpression of HER-2 protein is known to be a factor that has negative effects on prognosis regarding histological nuclear grade, lymph node metastasis, recurrence and survival rates.14 Albert et al. argued that patients who show no HR expression but overexpression of HER-2 protein are more vulnerable to local-systematic recurrence for 8 years.21 However, this study indicated that HER-2 expression has no relation to systematic recurrence, which is consistent with the study by Lee et al.5 p53 is a tumor suppressor gene located on the chromosome p17, and is related to cell cycle and DNA restoration; and the mutation of this gene is correlated with genetic instability.14 The relevance with regard to p53 mutations, Ki-67, histological grade and lymph node metastasis or survival rates has been reported by many studies, but the results have been conflicting, and this study revealed that there is no relation to systematic recurrence.10192324 Blood vessel and lymphovascular invasion of breast cancer has been shown to be related to recurrence in multiple studies.81924 Previous studies revealed that lymphovascular and blood vessel invasion is related to the total or disease-free survival period, and this study also indicated that lymphovascular and blood vessel invasion is a factor related to systematic recurrence.1125
- EIC, which looks like normal tissues around an infiltrative mass, is also known to be a risk factor with regard to local recurrence, but its relevance to local recurrence is unclear and its pathological physiology is also not widely known. A few studies have claimed that EIC is related to systematic or local recurrence, but this study revealed no relation to systematic recurrence.
- This study found that among patients that received breast conserving surgery for stage I breast cancer, blood vessel and lymphovascular invasion in post-operative pathological examination is related to systematic recurrence. Therefore, patients exhibiting lymphovascular and blood vessel invasion in post-operative pathological examination require more active treatment and should be subject to more rigorous tracking. However, this study featured a limited number of patients and therefore general principles cannot be extrapolated; thus, it is necessary to conduct a large-scale study on a greater number of patients.
DISCUSSION
- 1. Lee JB, Kim DH, Min BW, Ryu KW, Um JW, Kim AR, et al. Factors influencing the recurrence of breast cancer following modified radical mastectomy. J Korean Breast Cancer Soc 2001;4:128–135.Article
- 2. Park KH, Kim SI, Ko SS, Park BW, Lee KS. The pattern of systemic failure and factors influencing on the outcome after distant metastasis in breast cancer. J Korean Breast Cancer Soc 2003;6:109–116.Article
- 3. Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 2006;24:5652–5657.ArticlePubMed
- 4. Nuyten DS, Kreike B, Hart AA, Chi JT, Sneddon JB, Wessels LF, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res 2006;8:R62.ArticlePubMedPMC
- 5. Lee HS, Kwak BS, Son BH, Ahn SH. Prognostic factors influence on the systemic recurrence in axillary lymph node negative breast cancer. J Korean Surg Soc 2009;77:238–245.Article
- 6. Kang SH, Chung KY, Kim YS, Kim JH. Disease free survival and prognostic factors for patients with breast conserving surgery. J Korean Surg Soc 2004;67:274–278.
- 7. Paik NS, Noh WC, Bang HY, Hwang DY, Choi DW, Lee JI, et al. Recurrence following breast conserving therapy. J Korean Breast Cancer Soc 2000;3:64–75.Article
- 8. Yi OV, Lee JW, Kim HJ, Lim WS, Park EH, LEE JS, et al. Risk factors of local recurrence after breast conserving therapy in invasive breast cancer. J Breast Cancer 2009;12:302–308.Article
- 9. Kute TE, Russell GB, Zbieranski N, Long R, Johnston S, Williams H, et al. Prognostic markers in node-negative breast cancer: a prospective study. Cytometry B Clin Cytom 2004;59:24–31.ArticlePubMed
- 10. Brenin DR, Manasseh DM, El-Tamer M, Troxel A, Schnabel F, Ditkoff BA, et al. Factors correlating with lymph node metastases in patients with T1 breast cancer. Ann Surg oncol 2001;8:432–437.ArticlePubMed
- 11. Kuru B, Camlibel M, Gulcelik MA, Alagol H. Prognostic factors affecting survival and disease-free survival in lymph node-negative breast carcinomas. J Surg Oncol 2003;83:167–172.ArticlePubMed
- 12. Tanis E, van de Velde CJ, Bartelink H, van de Vijver MJ, Putter H, van der Hage JA. Locoregional recurrence after breast-conserving therapy remains an independent prognostic factor even after an event free interval of 10 years in early stage breast cancer. Eur J Cancer 2012;48:1751–1756.ArticlePubMed
- 13. Kim SI, Park BW, Lee KS. The impact of Patient age upon locoregional and systemic failures after breast conservation therapy: comparison of the results from the groups above and below 35 years. J Korean Breast Cancer Soc 2001;4:68–73.Article
- 14. Park SH, Kim SI, Park BW, Lee KS. Prognostic factors in axillary lymph node negative breast cancer. J Korean Breast Cancer Soc 2004;7:111–120.Article
- 15. Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five national surgical adjuvant breast and bowel project protocols of node-negative breast cancer. J Clin Oncol 2009;27:2466–2473.ArticlePubMedPMC
- 16. Van der Hage JA, Putter H, Bonnema J, Bartelink H, Therasse P, Van de Velde CJ, et al. Impact of locoregional treatment on the early-stage breast cancer patients: a retrospective analysis. Eur J Cancer 2003;39:2192–2199.ArticlePubMed
- 17. Touboul E, Buffat L, Belkacémi Y, Lefranc JP, Uzan S, Lhuillier P, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys 1999;43:25–38.ArticlePubMed
- 18. Kang HS, Noh DY, Youn YK, Oh SK, Choe KJ. The predictors of axillary node metastasis in 2cm or less breast cancer univariate and multivariate analysis. J Korean Breast Cancer Soc 1999;2:7–13.Article
- 19. Truong PT, Jones SO, Kader HA, Wai ES, Speers CH, Alexander AS, et al. Patients with T1 to T2 breast cancer with one to three positive nodes have higher local and regional recurrence risks compared with node-negative patients after breast-conserving surgery and whole-breast radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:357–364.ArticlePubMed
- 20. Son BH, Ahn SH, Kwak BS, Kim JK, Kim HJ, Hong SJ, et al. The recurrence rate, risk factors and recurrence patterns after surgery in 3700 patients with operable breast cancer. J Breast Cancer 2006;9:134–144.Article
- 21. Albert JM, Gonzalez-angulo AM, Guray M, Sahin A, Strom EA, Tereffe W, et al. Estrogen/progesterone receptor negativity and her2 positivity predict locoregional recurrence in patients with T1a, bN0 breast cancer. Int J Radiat Oncol Biol Phys 2010;77:1296–1302.ArticlePubMed
- 22. Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 2007;9:R6.ArticlePubMedPMC
- 23. Choi NK, Kim SY, Kim TY, Chae MK, Baek MJ, Lim CW, et al. Clinical correlation of c-erbB-2, p53, bcl-2, and c-myc expression in patients with breast cancer. J Korean Surg Soc 2002;62:371–380.Article
- 24. Touboul E, Buffat L, Belkacémi Y, Lefranc JP, Uzan S, Lhuillier P, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys 1999;43:25–38.ArticlePubMed
- 25. Noh JM, Choi DH, Huh SJ, Park W, Yang JH, Nam SJ, et al. Patterns of recurrence after breast-conserving treatment for early stage breast cancer by molecular subtype. J Breast Cancer 2011;14:46–51.ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 


 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE


 PubReader
PubReader Cite
Cite