Articles
- Page Path
- HOME > Kosin Med J > Volume 35(2); 2020 > Article
-
Original Articles
Efficacy of Evolocumab in Patients with Hypercholesterolemia - Xuan Jin, Moo Hyun Kim, Young-Rak Cho, Jong-Sung Park, Kai Song, Song Lin Yuan
-
Kosin Medical Journal 2020;35(2):125-132.
DOI: https://doi.org/10.7180/kmj.2020.35.2.125
Published online: December 31, 2020
Department of Cardiology, Dong-A University College of Medicine, Busan, Korea
- Corresponding Author: Moo Hyun Kim, Department of Cardiology, Dong-A University College of Medicine, 26, Daeshingongwon-ro, Seo-Gu, Busan 49201, Korea, Tel: +82-51-240-2976, Fax: +82-51-255-2177, E-mail: kimmh@dau.ac.kr
Copyright © 2020 by Korean Association of Medical Journal Editors
Articles published in Kosin Medical Journal are open-access, distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,531 Views
- 18 Download
- 1 Crossref
Abstract
-
Objectives
- The FOURIER trial reported that inhibition of PCSK9 with evolocumab on a background of statin therapy lowered low-density lipoprotein (LDL) cholesterol levels to a median of 30 mg per deciliter (0.78 mmol per liter) and reduced the risk of cardiovascular events. Here, we report data from a single center focusing on the effect of a PCSK9 inhibitor antibody on hyperlipidemia.
-
Methods
- We enrolled 29 hypercholesterolemia patients who had LDL cholesterol levels ≥ 70 mg per deciliter or non-HDL cholesterol ≥ 100 mg per deciliter and were divided into two groups (placebo n = 14, evolocumab n = 15), and participated in a 72 – 96 week, randomized, double-blind, placebo-controlled trial with statin therapy. Patients were randomly assigned to receive evolocumab (140 mg every 2 weeks or 420 mg monthly) or matched placebo via subcutaneous injection. Lipid changes during follow-up were analyzed.
-
Results
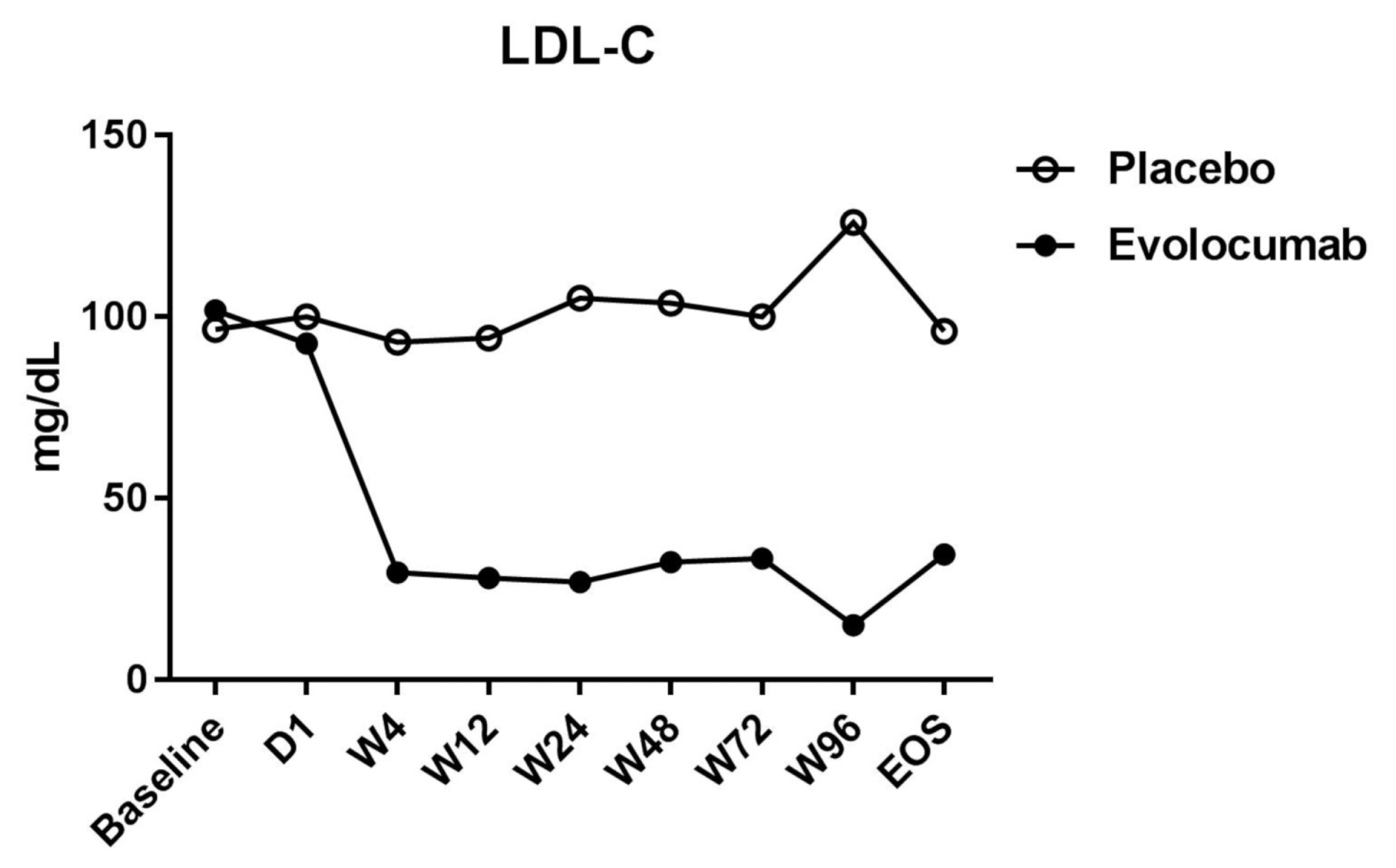
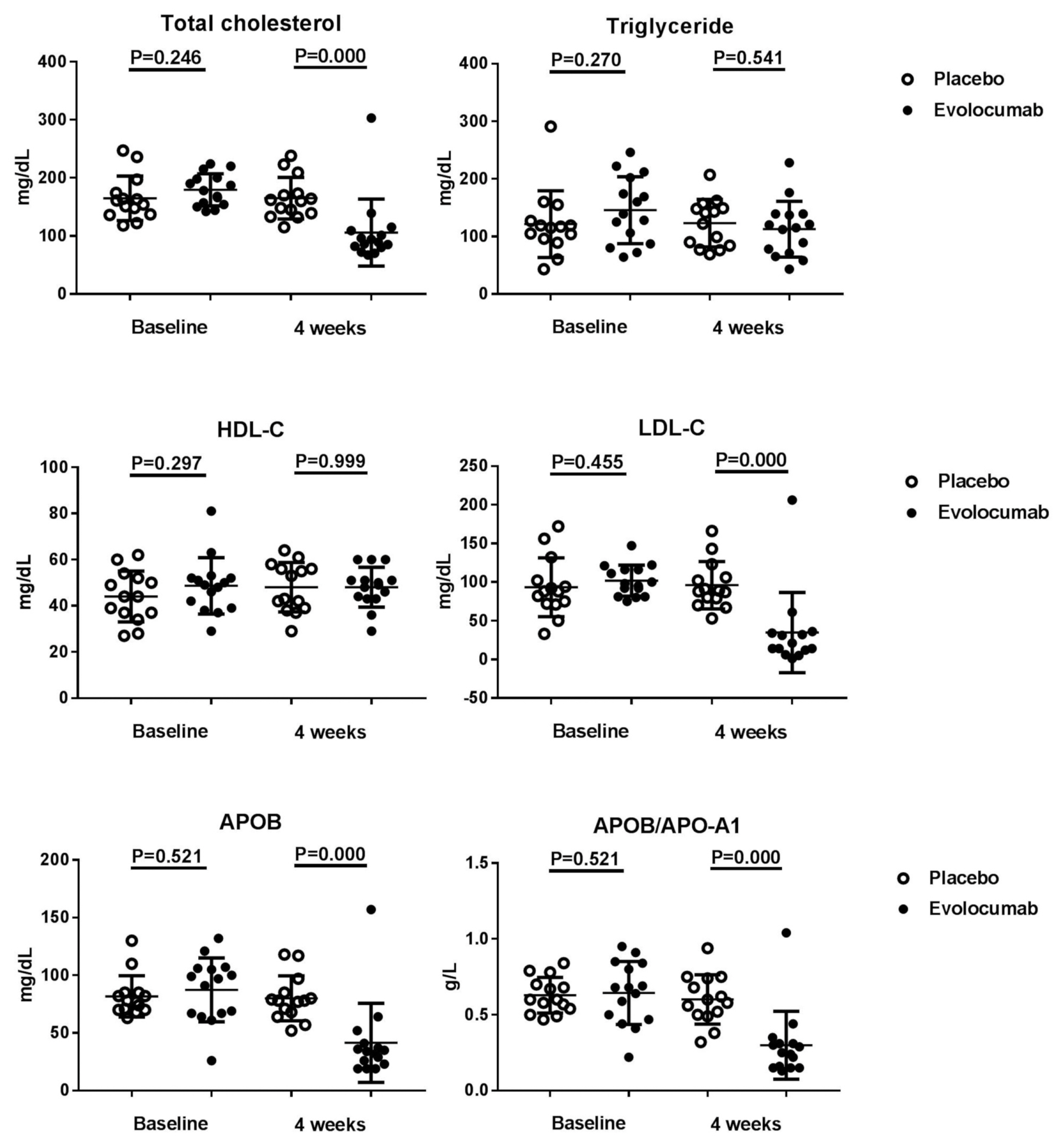
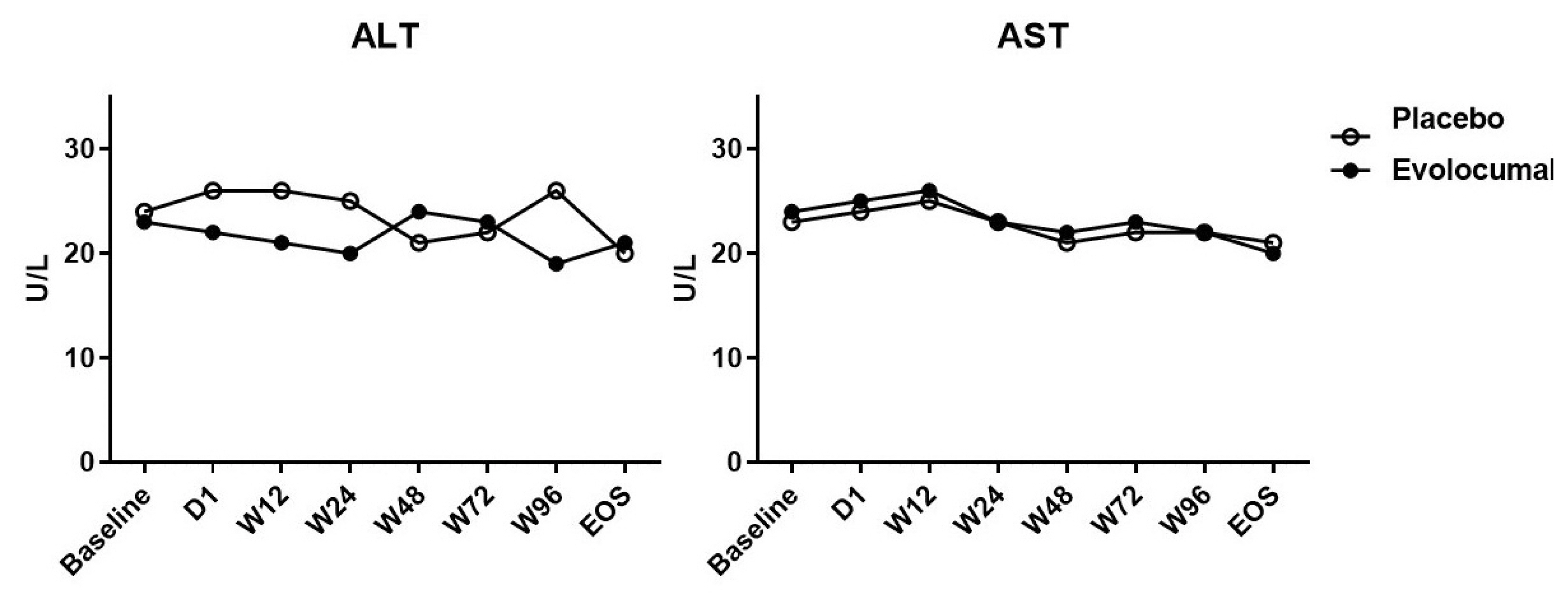
- The median LDL cholesterol level at baseline was 88 mg per deciliter, and the average LDL cholesterol level was 101.8 ± 20.0 mg per deciliter. At 4 weeks, the median LDL cholesterol level was 39 mg per deciliter, and the average LDL cholesterol level was 34.8 ± 51.8 mg per deciliter. Compared to placebo group, the LDL cholesterol levels were significantly reduced after treatment (P < 0.001), as well as total cholesterol, ApoB, and ApoB/ApoA1 levels. During follow-up, no discomfort was reported at local injection sites, and no cases of abnormal liver function were observed.
-
Conclusions
- Evolocumab significantly reduced LDL cholesterol levels and was well tolerated.
- A 72–96 weeks, randomized, double-blind, placebo-controlled (n = 14) study of evolocumab (n = 15) was conducted between July 2014 and May 2016 with 29 hypercholesterolemia patients who had LDL cholesterol ≥ 70 mg per deciliter or non-HDL cholesterol levels ≥ 100 mg per deciliter with statin therapy (moderate intensity was defined with atorvastatin 10–20 mg or rosuvastatin 5–10 mg; high intensity was defined with atorvastatin 40–80 mg or rosuvastatin 10–20 mg) in our single center, and no patients interrupted statin therapy. Patients were randomized equally and evolocumab or placebo was administered subcutaneously every 4 weeks and lipid changes were assessed. Eligible patients were randomly assigned in a 1:1 ratio to receive subcutaneous injections of evolocumab (either 140 mg every 2 weeks or 420 mg every month, according to patient preference) or matching placebo.
- Study visits were scheduled at screening and day 1, and then at weeks 2, 4, 8, and 12. Patients received subcutaneous evolocumab or placebo on day 1 and at weeks 4 and 8 (3 doses). Blood samples for all assessments were collected after an overnight fast (water only) and analyzed by a central laboratory. After screening, investigators, site staff, and study team members were blinded to all assessment results.
- The institutional review board or independent ethics committee at each site approved the protocol and informed consent form. All patients provided written informed consent before study procedures were performed.
- Statistical methods: Continuous variables are presented as mean ± standard deviation (SD) and categorical variables are presented as N (%). To determine whether differences between CAD cases and controls were significant for continuous and categorical variables, Student t-test and chi-squared test were used, respectively. All statistical analyses were two-sided and performed with SPSS (Version 22.0, SPSS Inc., Chicago IL, USA), with the threshold for significance set at P < 0.05 for all analyses performed.
MATERIALS AND METHODS
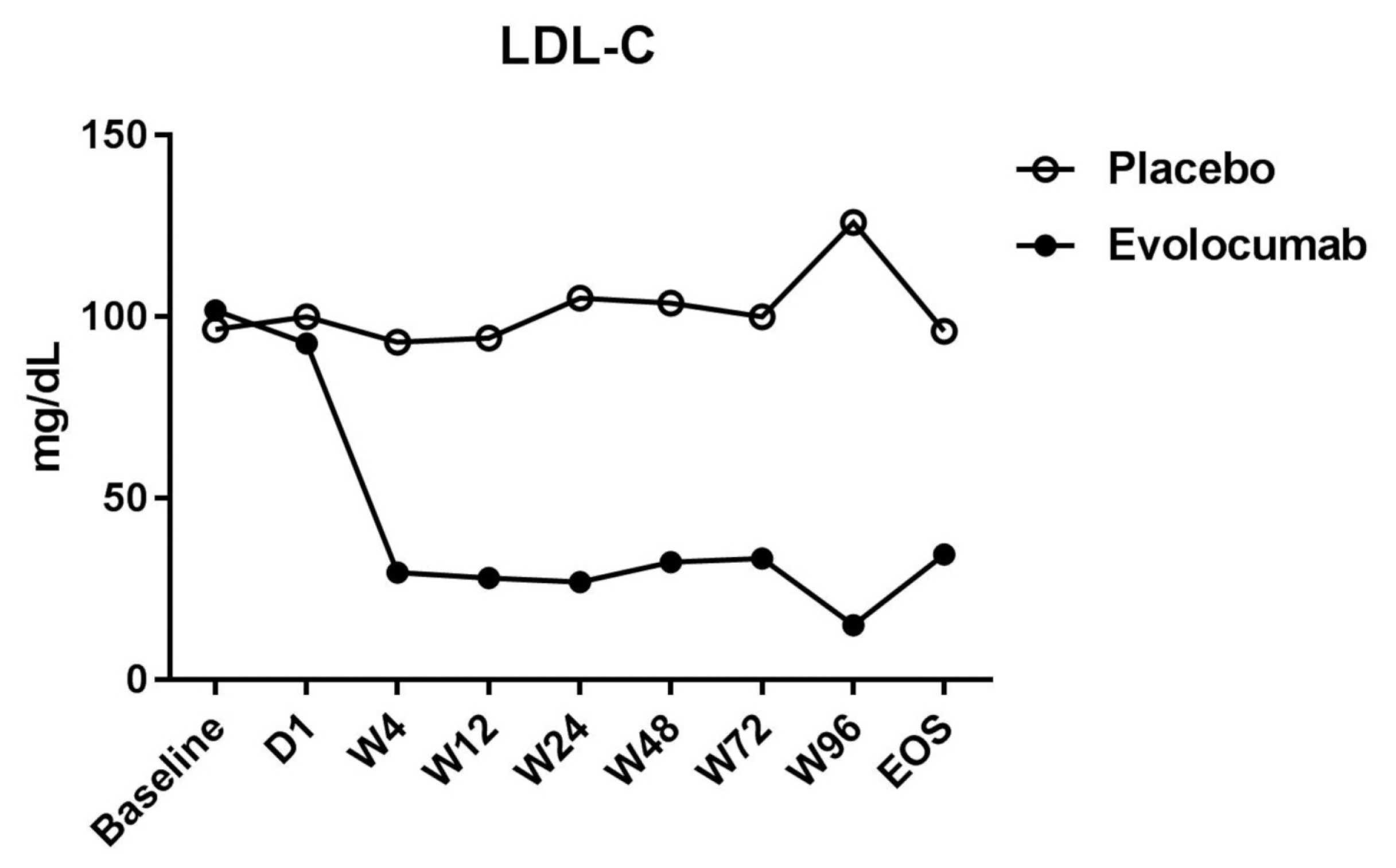
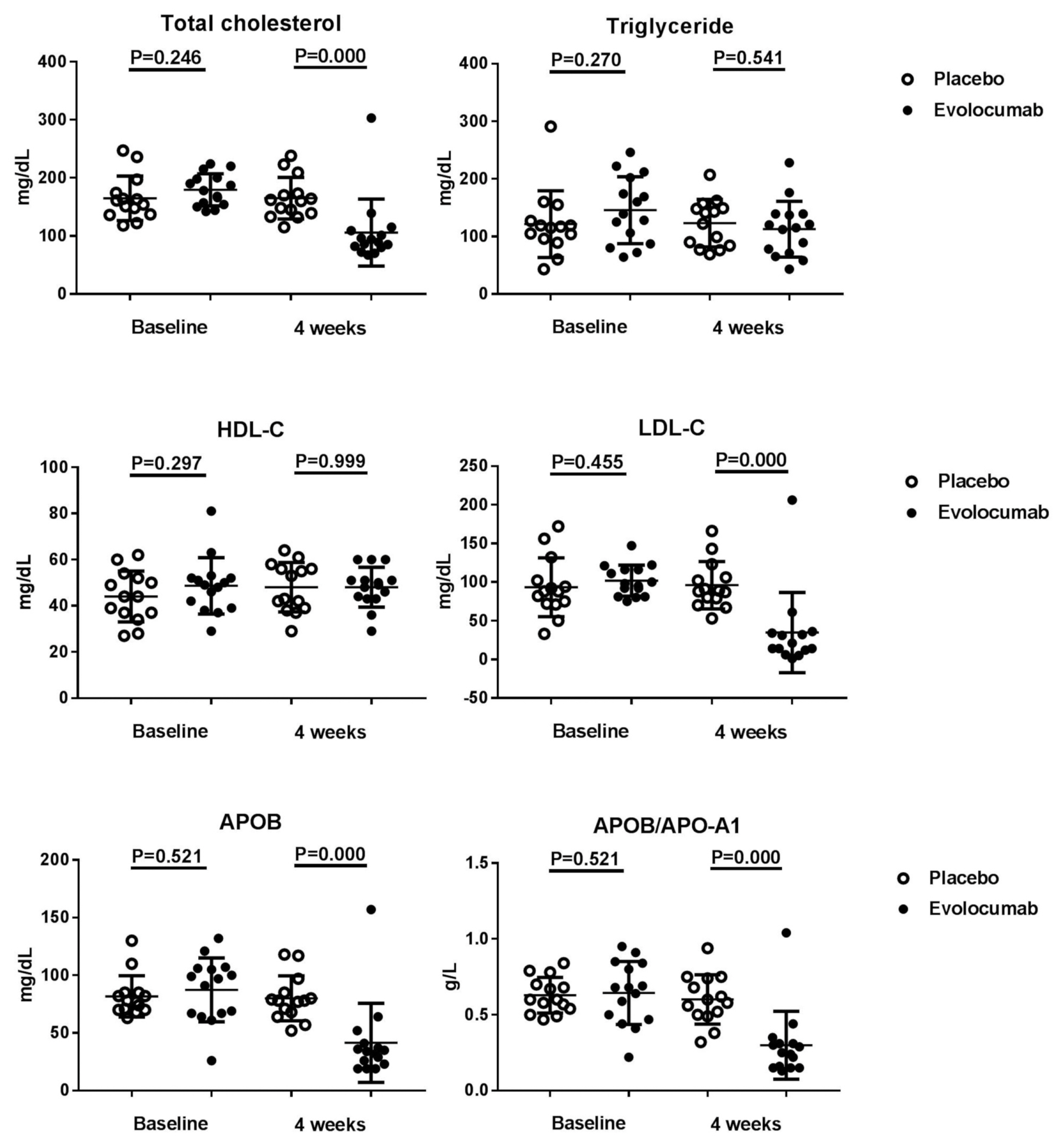
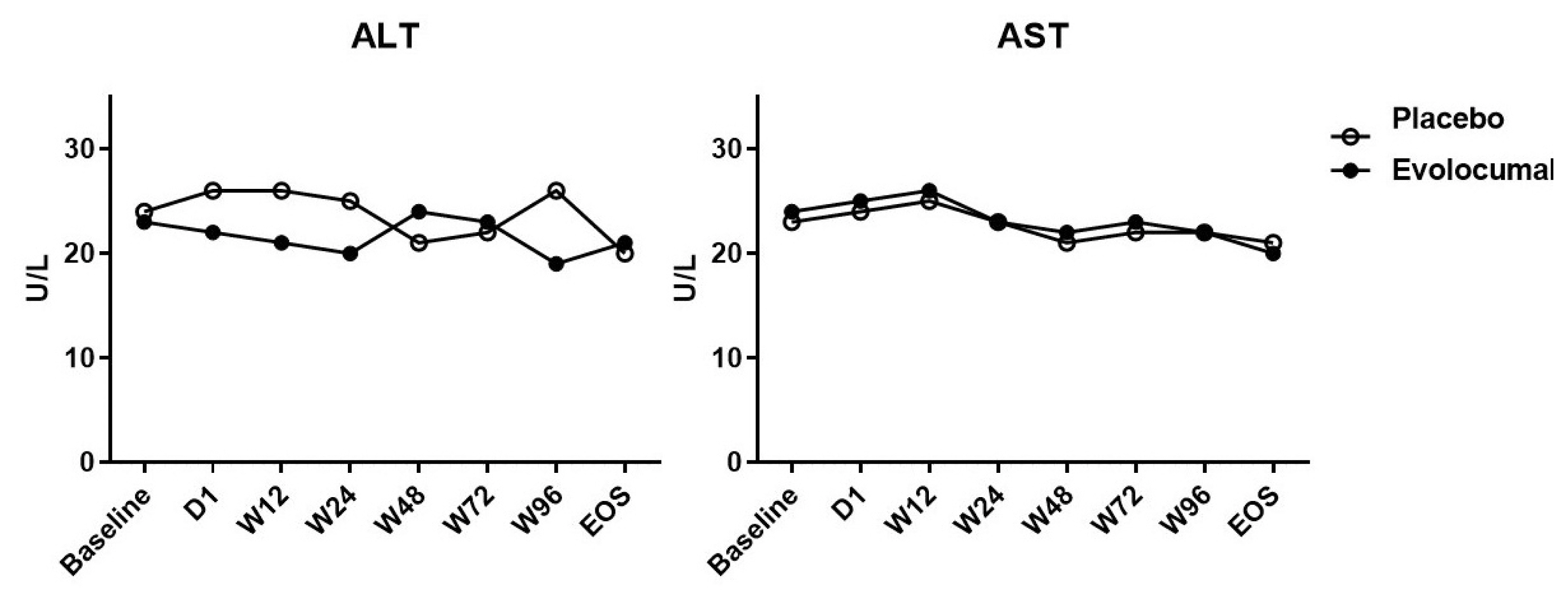
- Of the 36 patients screened, 29 were randomized (evolocumab n = 15; placebo n = 14) (Table 1). Seven patients did not meet the criteria and were excluded from the analyses. Table 1 shows that the patients’ characteristics at baseline were similar among the two groups, and most of the enrolled patients were men, but evolocumab group was older than placebo group, the reason may be caused by single center phenomenon. There was no significant difference in lipid levels between the placebo group and the evolocumab group before administration (Table 2). The median LDL cholesterol level at baseline was 88 mg per deciliter, and the average of LDL cholesterol level was 101.8 ± 20.0 mg per deciliter. At 4 weeks, the median LDL cholesterol level was 39 mg per deciliter, and the average of LDL cholesterol level was 34.8 ± 51.8 mg per deciliter, compared to placebo group the LDL cholesterol levels significantly decreased after treatment (P < 0.001) (Table 2) (Fig. 1). The median total cholesterol level at baseline was 187 mg per deciliter, and the average total cholesterol level was 179.5 ± 27.8 mg per deciliter. At 4 weeks, the median total cholesterol level was 80 mg per deciliter, and the average total cholesterol level was 105.9 ± 57.7 mg per deciliter, compared to placebo group total cholesterol levels significantly decreased after treatment (P < 0.001) (Table 2) (Fig. 2). The median ApoB level at baseline was 107 mg per deciliter, and the average ApoB level was 87.5 ± 27.7 mg per deciliter. At 4 weeks, the median ApoB level was 26 mg per deciliter, and the average ApoB level was 41.5 ± 34.3 mg per deciliter, compared to placebo group ApoB significantly decreased after treatment (P < 0.001) (Table 2) (Fig. 2). The median ApoB/ApoA1 level at baseline was 0.59 mg per deciliter, and the average for ApoB/ApoA1 was 0.6 ± 0.2 mg per deciliter. At 4 weeks, the median ApoB/ApoA1 level was 0.16 mg per deciliter, and the average for ApoB/ApoA1 was 0.3 ± 0.2 mg per deciliter, compared to placebo group the ApoB/ApoA1 level significantly decreased after treatment (P < 0.001) (Table 2) (Fig. 2). During follow-up, AST and ALT levels remained within the normal range, and no cases of abnormal liver function were found (Fig. 3).
RESULTS
- The FOURIER study15 revealed that evolocumab lowered LDL cholesterol levels by 59% from baseline levels as compared with placebo, from a median of 92 mg per deciliter (2.4 mmol per liter) to 30 mg per deciliter (0.78 mmol per liter). Our results showed that the median LDL cholesterol level in the placebo group was 88 mg per deciliter, and the average LDL cholesterol level of the placebo group was 101.8 ± 20.0 mg per deciliter. After treatment, the median LDL cholesterol level in the evolocumab group was 39 mg per deciliter, and the average LDL cholesterol level in the evolocumab group was 34.8 ± 51.8 mg per deciliter, with the evolocumab group showing reduced LDL cholesterol levels by 56% compared to the placebo group. The FOURIER study15 reported no significant differences in the overall rates of adverse events between the two groups, and injection site reactions were rare, although they were more frequent with evolocumab (2.1% vs. 1.6%). However, there were no adverse reactions during treatment and no injection site reactions observed in our study. The baseline statin dose for the patients in our study was lower than that used in the FOURIER study, as Asian patients are less likely to be prescribed with high-dose statins than their Caucasian counterparts.
- Statins are the most efficacious agents for alleviating LDL cholesterol levels, although some patients cannot tolerate treatment primarily due to muscle-related side effects, and sometimes higher doses are required to achieve target LDL cholesterol levels.16 Nevertheless, statin-intolerant patients require more effective LDL cholesterol-lowering therapies. Our findings suggest that evolocumab combined with statin therapy can effectively reduce lipid LDL-C levels.
DISCUSSION
Acknowledgments
ACKNOWLEDGEMENTS
Data are presented as mean ± SD or number (%).
BMI = body mass index; LVEF = left ventricular ejection fraction; HTN = hypertension; ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CCB = calcium channel blockers; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; ApoA1 = apolipoprotein A1; ApoB = apolipoprotein B.
Data are presented as mean ± SD.
AST = aspartate aminotransferase; ALT = alanine aminotransferase; HbA1c = hemoglobin A1c, Total-C = total cholesterol, other abbreviations as for Table 1.
- 1. Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A 2003;100:928–33.ArticlePubMedPMC
- 2. Abifadel M, Guerin M, Benjannet S, Rabès JP, Le Goff W, Julia Z, et al. Identification and characterization of new gain-of-function mutations in the PCSK9 gene responsible for autosomal dominant hypercholesterolemia. Atherosclerosis 2012;223:394–400.ArticlePubMed
- 3. Elagoz A, Benjannet S, Mammarbassi A, Wickham L, Seidah NG. Biosynthesis and Cellular Trafficking of the Convertase SKI-1/S1P Ectodomain Shedding Requires SKI-1 Activity. J Biol Chem 2002;277:11265–75.PubMed
- 4. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol 2015;65:2638–51.ArticlePubMed
- 5. Rousselet E, Marcinkiewicz J, Kriz J, Zhou A, Hatten ME, Prat A, et al. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J Lipid Res 2011;52:1383–91.ArticlePubMedPMC
- 6. Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154–6.ArticlePubMed
- 7. Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 2005;37:161–5.ArticlePubMed
- 8. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–72.ArticlePubMed
- 9. Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis 2007;193:445–8.ArticlePubMed
- 10. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014;370:1809–19.ArticlePubMed
- 11. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, et al. Effect of evolocumab or ezetimibe added to moderate-or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. Jama 2014;311:1870–83.ArticlePubMed
- 12. Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2531–40.PubMed
- 13. Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–8.PubMed
- 14. Raal FJ, Stein EA, Dufour R, Turmer T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:331–40.ArticlePubMed
- 15. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22.ArticlePubMed
- 16. Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. Jama 2012;308:2497–506.ArticlePubMed
References
Figure & Data
References
Citations

- Safety, adherence and efficacy of PCSK9 inhibitors: a retrospective real-world study
Bee Ling Kelly Chng, Wei Min Paul Heng, Yu Ming Soon, Jin Shing Hon, Yee How Lau, Ru San Tan, Jack Wei Chieh Tan
Proceedings of Singapore Healthcare.2022; 31: 201010582211441. CrossRef

 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite