Articles
- Page Path
- HOME > Kosin Med J > Volume 38(2); 2023 > Article
-
Original article
Intraoperative tumor localization using a titanium ring strip in totally laparoscopic distal gastrectomy for middle-third gastric cancer -
Jae-Kyun Park1,2
 , Chang-In Choi1,2
, Chang-In Choi1,2 , Tae Yong Jeon1,2
, Tae Yong Jeon1,2 , Hyuk Jae Jung1,2
, Hyuk Jae Jung1,2 , Si Hak Lee3
, Si Hak Lee3 , Sun Hwi Hwang3
, Sun Hwi Hwang3 , Dae-Hwan Kim1,2
, Dae-Hwan Kim1,2
-
Kosin Medical Journal 2023;38(2):126-133.
DOI: https://doi.org/10.7180/kmj.23.113
Published online: June 23, 2023
1Department of Surgery, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
2Medical Research Institute, Institute, Pusan National University Hospital, Busan, Korea
3Department of Surgery, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Busan, Korea
- Corresponding Author: Dae Hwan Kim, MD, PhD Department of Surgery, Pusan National University Hospital, Pusan National University School of Medicine, 179 Gudeok-ro, Seo-gu, Busan 49241, Korea Tel: +82-51-240-7238, Fax: +82-51-247-1365, E-mail: dh2-kim@hanmail.net
Copyright © 2023 Kosin University College of Medicine.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,348 Views
- 18 Download
Abstract
-
Background
- This study presents a novel technical tip for intraoperative tumor localization and determination of the proximal resection line using a titanium ring strip for totally laparoscopic distal gastrectomy in patients with middle-third gastric cancer and describes the short-term results of its application.
-
Methods
- In total, 42 patients with middle-third gastric cancer who underwent intraoperative tumor localization using a titanium ring strip and determination of the proximal resection line through intraoperative radiography between January 2020 and December 2021 were enrolled in this study. We retrospectively analyzed patients’ prospectively collected clinical, pathological, and surgical data.
-
Results
- Twenty-six men and 16 women with a mean age of 58.3±12.5 years were enrolled. The mean operation time and estimated blood loss were 212.6±43.0 minutes and 122.4±77.6 mL, respectively. The lengths of the proximal and distal resection margin were 2.0±0.4 cm (range, 0.8–3.7 cm) and 10.5±4.1 cm (range, 0.4–20.4 cm), respectively. Roux-en-Y anastomosis was performed in 30 patients, while Billroth II with Braun anastomosis was performed in 12 patients. There were no procedure-related complications, and the mean postoperative hospital stay was 7.2±1.9 days. For all patients, the negative proximal resection margin was confirmed by postoperative pathological examinations.
-
Conclusions
- Intraoperative tumor localization and determination of the proximal resection line using a titanium ring strip is a useful alternative method that can be easily and safely performed. This method is especially useful for patients with middle-third gastric cancer requiring an appropriate proximal resection margin.
- When performing gastrectomy in gastric cancer, it is crucial to identify the proximal border of cancer and to determine the appropriate proximal resection line, as curative gastric resection requires complete resection of the primary lesion with a safety margin and radical lymphadenectomy [1]. Tumor localization and determination of the proximal resection line are particularly important for patients with middle-third gastric cancer. Because excessive gastric resection for a sufficient proximal resection margin may reduce the residual gastric volume more than necessary, resulting in a decrease in postoperative quality of life (QOL), and conversely, insufficient gastric resection to minimize residual gastric volume loss may not secure a negative resection margin [2-4]. Therefore, in middle-third gastric cancer, it is more important to secure an “appropriate” resection margin rather than a “sufficient” resection margin.
- In advanced gastric cancer (AGC), the location of the tumor can be confirmed visually or through palpation because the cancer invades the serosa or its large size. However, it is difficult to apply this method in early gastric cancer (EGC), so the location of the tumor is indirectly estimated by preoperative endoscopic clipping and confirming the clip via palpation during operation. In the early days of laparoscopic gastrectomy, laparoscopy-assisted gastrectomy was mainly performed. With the recent development of surgical techniques and instruments and the growing interest in postoperative QOL, totally laparoscopic gastrectomy (TLG) is increasingly performed [5,6]. In laparoscopy-assisted gastrectomy, tumor localization and determination of the proximal resection line were performed through traditional preoperative endoscopic clipping and intraoperative palpation. However, this method is difficult to apply for TLG because surgeon cannot palpate the lesion or endoclip manually, and such difficulty must be overcome when performing TLG.
- Many researchers have described various intraoperative tumor localization and proximal resection line determination methods that can be used when performing TLG [7-13]. However, while such methods have advantages, they also have obvious disadvantages because an endoscope must be used during surgery or special equipment such as laparoscopic ultrasound may be required, so there is no standardized method yet. Since we have implemented another method that can compensate for the shortcomings of the methods reported so far, we conducted this study to introduce the technical tips and short-term outcomes of our method.
Introduction
- Ethical statements: This study was approved by the Institutional Review Board of Pusan National University Hospital (IRB No. 2302-006-123) and was conducted in accordance with the recent Declaration of Helsinki. Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
- 1. Patients
- Between January 2020 and December 2021, 368 patients underwent TLG for lower- or middle-third EGC at our institution. Of these 368 patients, 42 patients whose endoscopic proximal border of the lesion was located between 5 cm distal from the gastro-esophageal junction and 2 cm proximal from the gastric angle, were underwent intraoperative tumor localization and determination of the proximal resection line using titanium ring strip. We retrospectively analyzed the clinical, pathological, and surgical data.
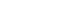
- 2. Preparation of the titanium ring strip
- The structure of the titanium ring and the preparation process of titanium ring strip are shown in Fig. 1. The titanium ring was custom-made for this procedure and had a diameter of 10 mm with a 5 mm hole in the center. The titanium ring strip was constructed by fixing the titanium ring to the umbilical tape via suture ligation. Each of the three metal rings was fixed at 1.5-cm intervals for the lesser curvature side and at 2.5-cm intervals for the greater curvature side. A suture ligation was performed between titanium rings and umbilical tape to prevent separation.
- 3. Surgical procedure
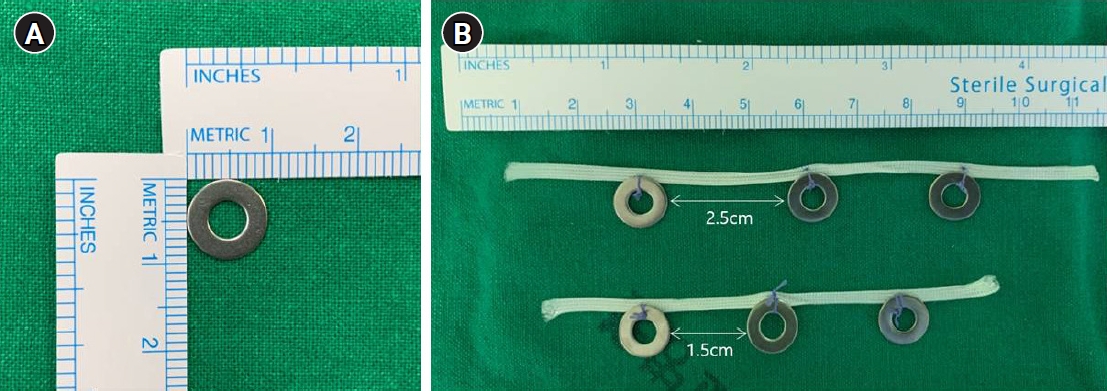
- For all patients, endoscopic ultrasonography was performed the day before surgery to measure the depth of invasion, and two endoscopic clips were placed 1 cm proximal of the macroscopic proximal border of the lesion (Fig. 2). Under general anesthesia, TLD was performed using the traditional five-trocar method or a reduced port method using three trocars. In all patients, total omentectomy and D2 lymph node dissection (LND) were performed. While ligating the left gastroepiploic artery, the right gastroepiploic artery, and the right gastric artery, stations 4sb, 4d, 6, and 5 LND were performed. The duodenum was then transected using a linear laparoscopic stapler approximately 2 cm distal to the pyloric ring. Along the common hepatic artery, the proper hepatic artery, and the proximal splenic artery, stations 8a, 12a, and 11p LND were performed and then stations 7 and 9 LND were performed while ligating the left gastric artery. If necessary one or two short gastric artery ligation was performed.
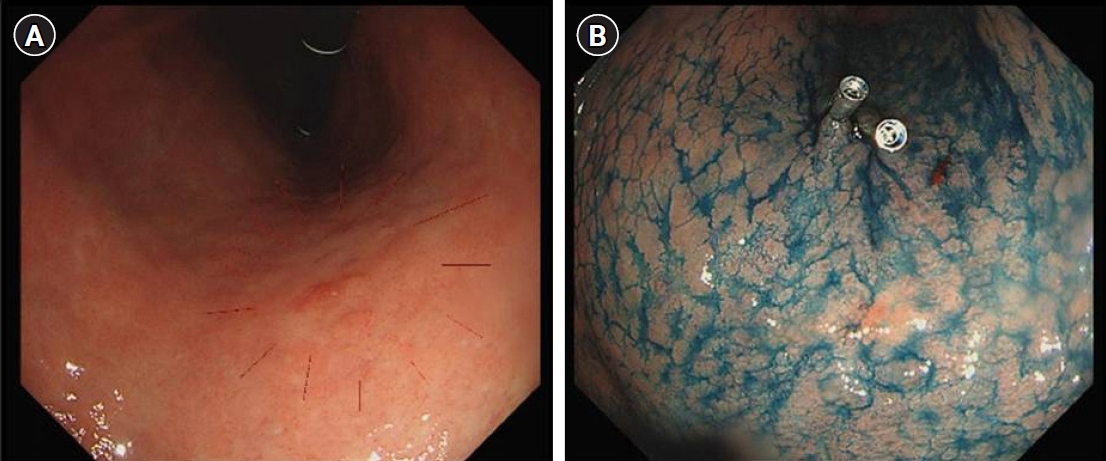
- After the D2 LND, we performed the intraoperative tumor localization and determination of the proximal resection line using a titanium ring strip. The pre-made titanium ring strip was inserted through a 12-mm port, the first ring of the 1.5-cm interval titanium ring strip was placed on the right side of the gastro-esophageal junction, and the third ring of the 2.5-cm interval titanium ring strip was placed where the left gastroepiploic artery enters the gastric wall (Fig. 3A). And then the positional relationship between the endoscopic clip and the titanium ring was identified using portable abdominal radiography. (Fig. 3B) On this basis, the proximal resection line in the actual surgical field was designed at the 1 cm proximal of the endoscopic clip to secure the 2 cm proximal resection margin (Fig. 3C). To secure negative proximal resection margin and appropriate residual gastric volume, we aimed for a proximal resection margin of 1.5 to 2.5 cm.
- The stomach was resected using linear laparoscopic stapler, and the resected stomach was delivered through a mini-laparotomy made by a vertical extension of the umbilical incision. The negative proximal resection margin was confirmed via intraoperative frozen section biopsy prior to the anastomosis. Gastrointestinal continuity was restored by Roux-en-Y or Billroth II with Braun anastomosis. Gastrotomy and jejunotomy were performed using electrocautery, each arm of the linear laparoscopic stapler was inserted into the stomach and jejunum, and the side-to-side gastrojejunostomy and jejunojejunostomy were performed by firing the linear laparoscopic stapler. The common entry hole was closed manually.
Methods
- The clinicopathological and operative data of the 42 patients are summarized in Table 1. There were 26 males and 16 females, with a mean age of 58.3±12.5 years (range, 23–87 years). The mean body mass index was 24.7±2.7 kg/m2 (range, 20.0–32.7 kg/m2), and the American Society of Anesthesiologists scores were I, II, and III in 8, 30, and 4 cases, respectively. The mean operation time and estimated blood loss was 212.6±43.0 minutes (range, 160–300 minutes) and 122.4±77.6 mL (range, 50–300 mL), respectively.
- Roux-en-Y anastomosis and Billroth II with Braun anastomosis was performed in 30 and 12 cases. The location of the lesion was in the lesser curvature, greater curvature, anterior wall, and posterior wall in 4, 19, 6, and 13 cases, respectively. The mean number of lymph node recovered was 38.3±13.0 (range, 17–69) and the postoperative pathological stages were IA, IB, and II in 32, 8, and 2 cases, respectively. The mean postoperative hospital stay was 7.2±1.9 days (range, 6–13 days) and there were no operation-related complications.
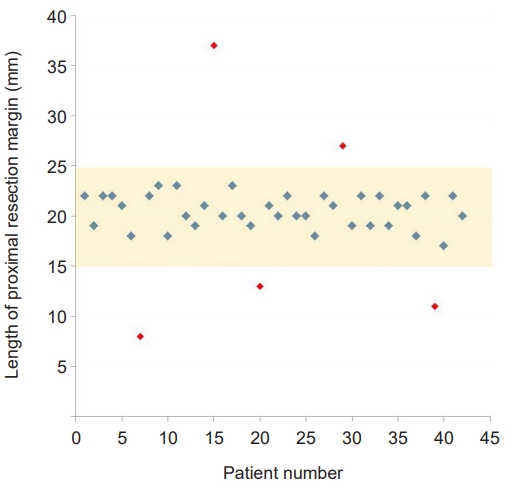
- The lengths of the proximal and distal resection margin were 2.0±0.4 cm (range, 0.8–3.7 cm) and 10.5±4.1 cm (range, 4.1–20.4 cm), respectively. The proximal resection margin was targeted at 1.5 to 2.5 cm, and as a result 37 cases (88.1%) had a proximal resection margin of 1.5 to 2.5 cm, but it was less than 1.5 cm in three cases (7.1%) and longer than 2.5 cm in two cases (4.8%) (Fig 4). Table 2 provides a summary of the lengths of the proximal and distal margins based on the location of the lesion. The deviation of the measured length according to the location was not significant.
Results
- As many studies have proven that the 5-year survival rate of laparoscopic gastrectomy in EGC has no difference from that of open gastrectomy, laparoscopic gastrectomy is recognized as a standard treatment for EGC beyond the indication of endoscopic resection, and its indication is expanding to AGC [14,15]. The curative gastric resection is composed of complete resection of the primary lesion with safety margin and radical lymphadenectomy. In patients with lower-third gastric cancer, remnant gastric volume and proximal resection margin can be sufficiently secured with the conventional 60% to 65% distal gastrectomy. However, in patients with middle-third gastric cancer, to avoid postoperative QOL reduction and negative proximal resection margin acquisition failure, it is more important to secure an “appropriate” proximal resection margin rather than a “sufficient” proximal resection margin via accurate tumor localization and determination of the proximal resection line. To this end, we aimed for a proximal resection margin of 1.5 to 2.5 cm, and as a result 37 cases (88.1%) had a proximal resection margin of 1.5 to 2.5 cm, but it was less than 1.5 cm in three cases (7.1%) and longer than 2.5 cm in two cases (4.8%). The causes of the five failed cases are presumed to be related to tissue shrinkage following formalin fixation, difference in the degree of infiltration according to the histological type of cancer.
- Laparoscopic gastrectomy has advantages such as minimal invasiveness, shortened length of hospital stay, and reduced postoperative pain. With the development of laparoscopic surgical techniques and equipments, various methods have recently been attempted to maximize the advantages of laparoscopic gastrectomy and improve the postoperative QOL of the patient, such as reduced port gastrectomy, single-port gastrectomy, and TLG [16-19]. TLG can maximize cosmetic effects by moving the location of the mini-laparotomy to the umbilicus. However, both gastrectomy and reconstruction are performed intracorporeally, tumor localization and determination of the proximal resection line via preoperative endoscopic clipping and intraoperative palpation is not possible.
- To overcome the shortcomings encountered during TLG, various methods have been reported. These methods can be divided into methods that require preoperative endoscopic clipping and methods that do not. Hyung et al. [8] reported a method for checking preoperative endoscopic clips by intraoperative laparoscopic ultrasonography. Although the operator directly performs a laparoscopic ultrasonography without the help of a radiologist, it requires expensive equipment, and if the operator lacks experience, it may be difficult to find an endoscopic clip located on the posterior wall of the stomach. Xuan et al. [19] and Park et al. [15] reported a tumor localization method through intraoperative endoscopy. This method had the advantage of being able to assess the location of the lesion more accurately in real time, but presented shortcomings including the requirement of endoscopic equipment, cooperation with the endoscopist, or endoscopy training for surgeons. In addition, gas injected into the stomach during endoscopy may expand the stomach and intestinal tract, making surgery difficult. Kim et al. [16] reported a method of simultaneously performing a preoperative endoscopic clip and metallic laparoscopic vascular clip applied to lesser and greater curvature, and determining the resection line by comparing the positions of the clips. This method was simple and easy to perform, thus we used it initially. However, our experience showed that metallic laparoscopic vessel clips sometimes caused bleeding or hematoma in the stomach wall or omentum. Furthermore, if the metallic laparoscopic vessel clips were located in the distal part of the lesion, an additional portable abdomen was required, resulting in a longer operation time. Recently, tumor localization using magnetic marking and RFID clips is being studied, but it is still in the animal testing stage and commercialization of the machine has yet to progress [11,12,20].
- A representative method that does not require preoperative endoscopic clipping is endoscopic tattooing. This method has the advantage of not requiring intraoperative portable abdominal radiography and preoperative endoscopic clipping, but it has some shortcomings, such as requiring co-work with an endoscopist or endoscopy training for surgeons. Our method also has the similar shortcomings of not being able to receive real-time feedback on the location of the lesion, requiring preoperative endoscopic clipping and intraoperative portable abdominal radiography. However, compared to the aforementioned methods, our method has several advantages. First, it does not require additional expensive equipment and can be easily performed without any specific training. Second, lesions located on the posterior wall of the stomach can be localized accurately. The stomach has a thicker wall than other hollow viscera, thus if the lesion is located on the posterior wall of the stomach, sometimes accurate localization may be difficult even with laparoscopic ultrasonography, magnetic marking clip, and RFID clip. In this study, a proximal resection margin of 1.5 to 2.5 cm was secured in all 13 patients with lesions in the posterior wall of the stomach. Third, since each of the three metal rings on the left and right sides of the stomach is compared with the intragastric clip, it is possible to more accurately determine the proximal resection line without unnecessary gastric serosa injury. In addition, the first ring on the lesser curvature side is usually positioned at the gastro-esophageal junction and the third ring on the greater curvature side is usually where the left gastroepiploic artery enters the stomach wall. Thus the location of all lesions in the middle-third of the stomach can be identified by a single portable abdominal radiography. Finally, this method has the advantage that it can be used conveniently in single-port gastrectomy or reduced-port gastrectomy. There were no cases of surgical complications related to this procedure but one technical error occurred. In the first case, titanium ring was separated from the umbilical tape when inserted through the trocar. So suture ligation should be performed when preparing the titanium ring strip as it may be difficult to detect or remove if the separated titanium ring moves between mesenteries or splenic bed.
- Despite these advantages, there are some limitations in this study. First, this study was a retrospective single-arm study, and as there was no control group for intervention, it was not possible to confirm how effective this method was compared to other methods. However, this study confirmed the clinical feasibility as a pilot study for the new technique, laying the groundwork for further studies that can compare it with other localization strategies. Second, there is a criterion for locating the titanium clip, but it is somewhat difficult to precisely determine the margin because the operator determines it roughly with the naked eye through the X-ray. However, given the ongoing debates about the ideal safety margin length in AGC, our approach in this study was deemed acceptable.
- In conclusion, our method also has shortcomings, such as the lack of real-time feedback on the location of the lesion, which requires preoperative endoscopic clipping and intraoperative portable abdominal radiography. But intraoperative tumor localization and determination of the proximal resection line using a titanium ring strip is an easy and safe alternative to other tumor localization methods. It can compensate for the shortcomings of the other methods reported to date, and it is especially useful for patients with middle-third gastric cancer requiring an appropriate proximal resection margin.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
This work was supported by a 2-Year Research Grant of Pusan National University.
-
Author contributions
Conceptualization: SHL, DHK. Data curation: CIC, TYJ, SHL, SHH, DHK. Formal analysis: JKP, CIC, HJJ, SHL. Methodology: JKP, HJJ, SHH. Funding acquisition: DHK. Project administration: TYJ. Supervision: CIC, TYJ, SHH. Writing - original draft: JKP. Writing - review and editing: CIC, HJJ, DHK.
Article information
- 1. Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer 2014;14:87–104.ArticlePubMedPMC
- 2. Kim A, Lee JB, Ko Y, Park T, Jo H, Jang JK, et al. Larger remaining stomach volume is associated with better nutrition and muscle preservation in patients with gastric cancer receiving distal gastrectomy with gastroduodenostomy. J Gastric Cancer 2022;22:145–55.ArticlePubMedPMCPDF
- 3. Heneghan HM, Yimcharoen P, Brethauer SA, Kroh M, Chand B. Influence of pouch and stoma size on weight loss after gastric bypass. Surg Obes Relat Dis 2012;8:408–15.ArticlePubMed
- 4. Braghetto I, Cortes C, Herquinigo D, Csendes P, Rojas A, Mushle M, et al. Evaluation of the radiological gastric capacity and evolution of the BMI 2-3 years after sleeve gastrectomy. Obes Surg 2009;19:1262–9.ArticlePubMedPDF
- 5. Noshiro H, Ohuchida K, Kawamoto M, Ishikawa M, Uchiyama A, Shimizu S, et al. Intraabdominal Roux-en-Y reconstruction with a novel stapling technique after laparoscopic distal gastrectomy. Gastric Cancer 2009;12:164–9.ArticlePubMedPDF
- 6. Takaori K, Nomura E, Mabuchi H, Lee SW, Agui T, Miyamoto Y, et al. A secure technique of intracorporeal Roux-Y reconstruction after laparoscopic distal gastrectomy. Am J Surg 2005;189:178–83.ArticlePubMed
- 7. Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol 2019;5:506–13.ArticlePubMedPMC
- 8. Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, et al. Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS-02-RCT randomized clinical trial. J Clin Oncol 2020;38:3304–13.ArticlePubMed
- 9. Ahn SH, Son SY, Jung DH, Park DJ, Kim HH. Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg 2014;219:933–43.ArticlePubMed
- 10. Kunisaki C, Makino H, Yamaguchi N, Izumisawa Y, Miyamato H, Sato K, et al. Surgical advantages of reduced-port laparoscopic gastrectomy in gastric cancer. Surg Endosc 2016;30:5520–8.ArticlePubMedPDF
- 11. Inaki N, Tsuji T, Doden K, Sakimura Y, Tawara H, Matsui R, et al. Reduced port laparoscopic gastrectomy for gastric cancer. Transl Gastroenterol Hepatol 2016;1:38.ArticlePubMedPMC
- 12. Inaki N. Reduced port laparoscopic gastrectomy: a review, techniques, and perspective. Asian J Endosc Surg 2015;8:1–10.ArticlePubMed
- 13. Luigiano C, Ferrara F, Morace C, Mangiavillano B, Fabbri C, Cennamo V, et al. Endoscopic tattooing of gastrointestinal and pancreatic lesions. Adv Ther 2012;29:864–73.ArticlePubMedPDF
- 14. Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Song SY, et al. Intraoperative tumor localization using laparoscopic ultrasonography in laparoscopic-assisted gastrectomy. Surg Endosc 2005;19:1353–7.ArticlePubMedPDF
- 15. Park DJ, Lee HJ, Kim SG, Jung HC, Song IS, Lee KU, et al. Intraoperative gastroscopy for gastric surgery. Surg Endosc 2005;19:1358–61.ArticlePubMedPDF
- 16. Kim HI, Hyung WJ, Lee CR, Lim JS, An JY, Cheong JH, et al. Intraoperative portable abdominal radiograph for tumor localization: a simple and accurate method for laparoscopic gastrectomy. Surg Endosc 2011;25:958–63.ArticlePubMedPDF
- 17. Ohdaira T, Nagai H. Intraoperative localization of early-stage upper gastrointestinal tumors using a magnetic marking clip-detecting system. Surg Endosc 2007;21:810–5.ArticlePubMedPDF
- 18. Joo HY, Lee BE, Choi CI, Kim DH, Kim GH, Jeon TY, et al. Tumor localization using radio-frequency identification clip marker: experimental results of an ex vivo porcine model. Surg Endosc 2019;33:1441–50.ArticlePubMedPDF
- 19. Xuan Y, Hur H, Byun CS, Han SU, Cho YK. Efficacy of intraoperative gastroscopy for tumor localization in totally laparoscopic distal gastrectomy for cancer in the middle third of the stomach. Surg Endosc 2013;27:4364–70.ArticlePubMedPDF
- 20. Kojima F, Sato T, Tsunoda S, Takahata H, Hamaji M, Komatsu T, et al. Development of a novel marking system for laparoscopic gastrectomy using endoclips with radio frequency identification tags: feasibility study in a canine model. Surg Endosc 2014;28:2752–9.ArticlePubMedPDF
References
Figure & Data
References
Citations


 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite