Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
Article information
Abstract
Abstract
Objectives
The aim of this study was whether quercetin induces cell death by caspase and MAPK signaling pathway in EGFR mutant lung cancer cells
Methods
PC-9 cells, EGFR mutant lung cancer cells, were treated various times and concentrations of quercetin and harvested and measured using MTT assay, DNA fragmentation, Western blotting, and FACS analysis.
Results
Treatment with quercetin in PC-9 cells resulted in inhibition of cell growth through apoptosis. Quercetin-induced apoptosis was associated with caspase-dependent manner. Quercetin also significantly increased levels of phosphor-p38 and decreased levels of phosphor-ERK, indicating that quercetin induces p38 MAPK signaling pathway in PC-9 cells. Quecetin treatment also generated the release of cytochrome c in PC-9 cells; however, pretreatment with rotenone or z-LEHD-fmk, significantly attenuated quercetin-induced apoptosis.
Conclusions
Our data indicate that quercetin exhibits EGFR mutant lung cancer effects through apoptosis by caspase dependent and mitochondrial pathway.
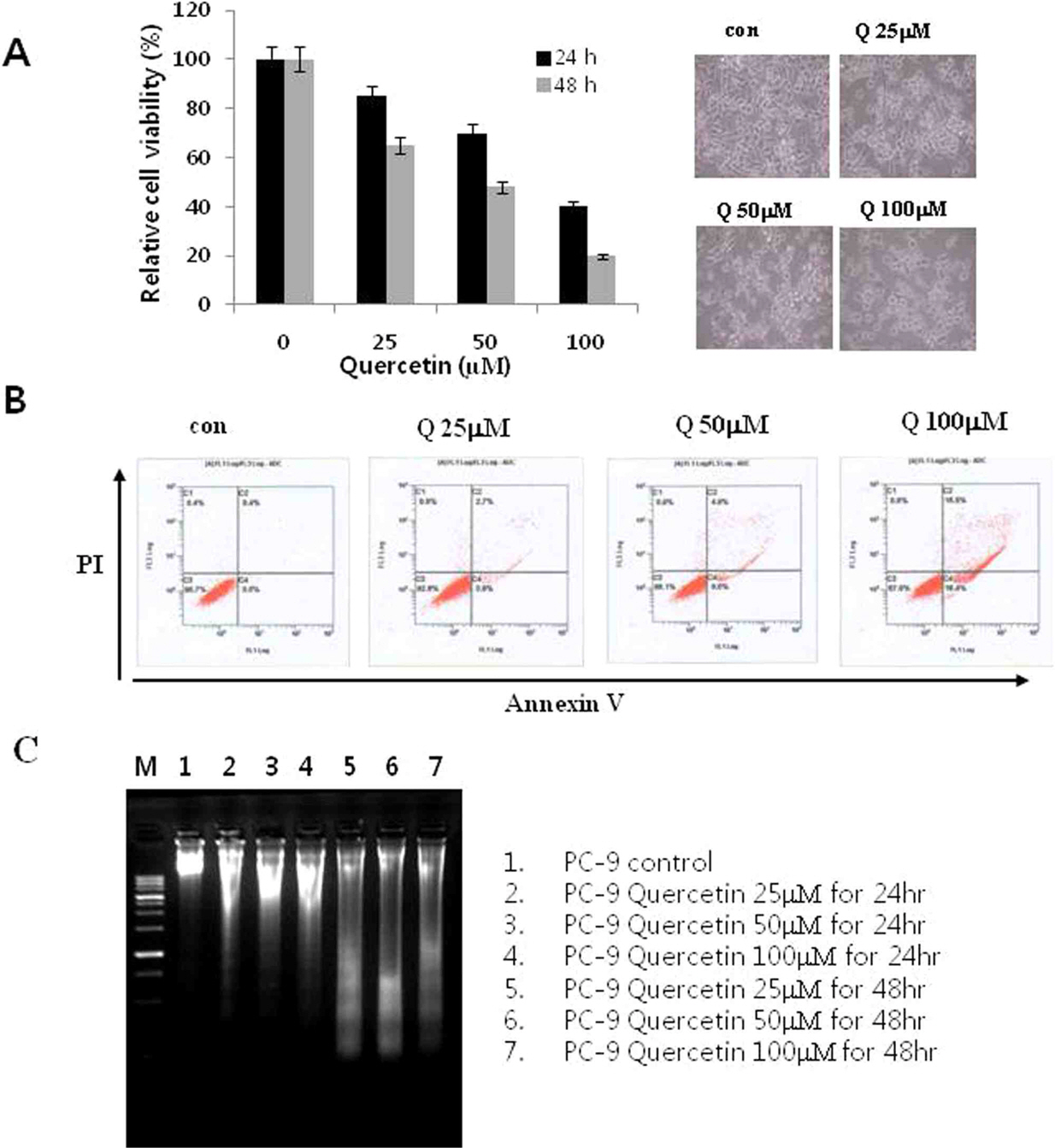
Effects of quercetin on cell growth and cell death in PC-9 cells. A. The growth inhibition was measured by MTT assay. B. The cell death distribution was analyzed by flow cytometry. C. After incubation with indicated concentrations of quercetin for 24 h, cells were collected and DNA was isolated. Con: control, Q: quercetin, PI: propidium iodide.
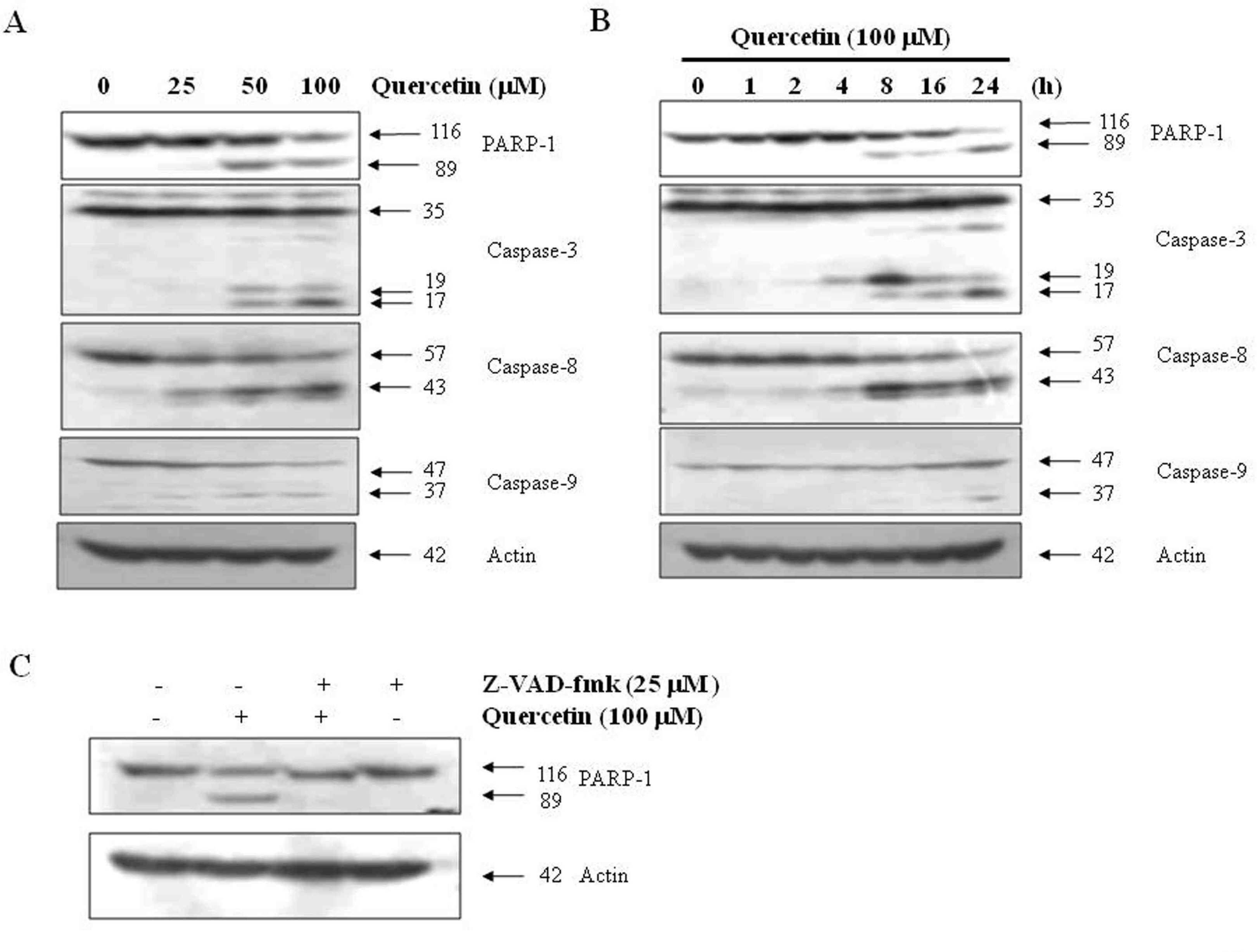
Effects of quercetin on caspase activation. A. PC-9 cells were treated for 24 h with the indicated concentrations of quercetin. B. PC-9 cells were treated 100 uM quercentin with the indicated various time. C. PC-9 cells were treated with 100 uM quercetin in the presence or absence of pan-caspase inhibitor (z-VAD-fmk) for 24 hr.
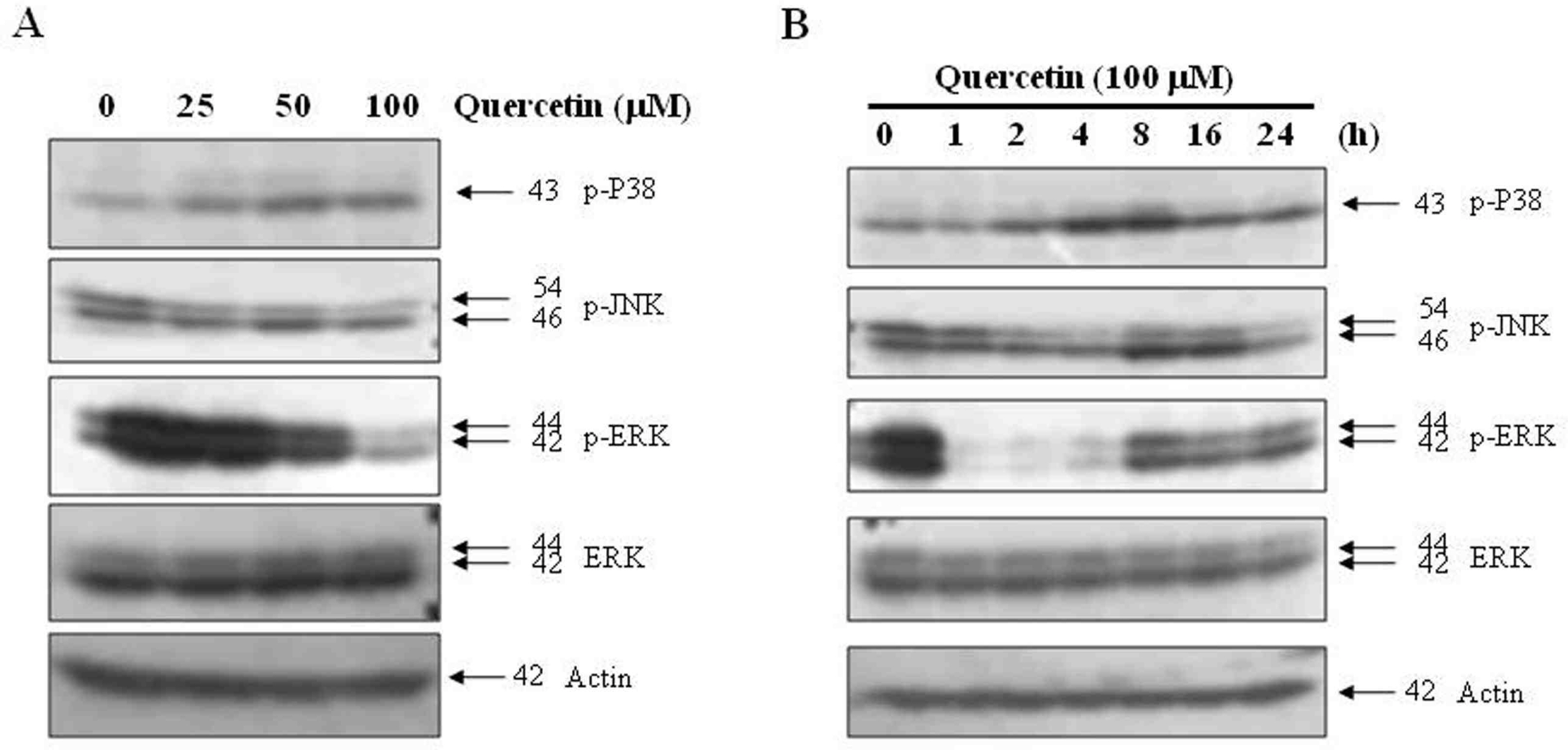
Effects of quercetin on the activation of MAPK in PC-9 cells. PC-9 cells were treated with 100 uM quercetin for the indicated concetrations (A) and times (B). The cell extracts were prepared for Western blot analysis of p-p38, p-JNK, p-ERK. Actin was used as an internal control
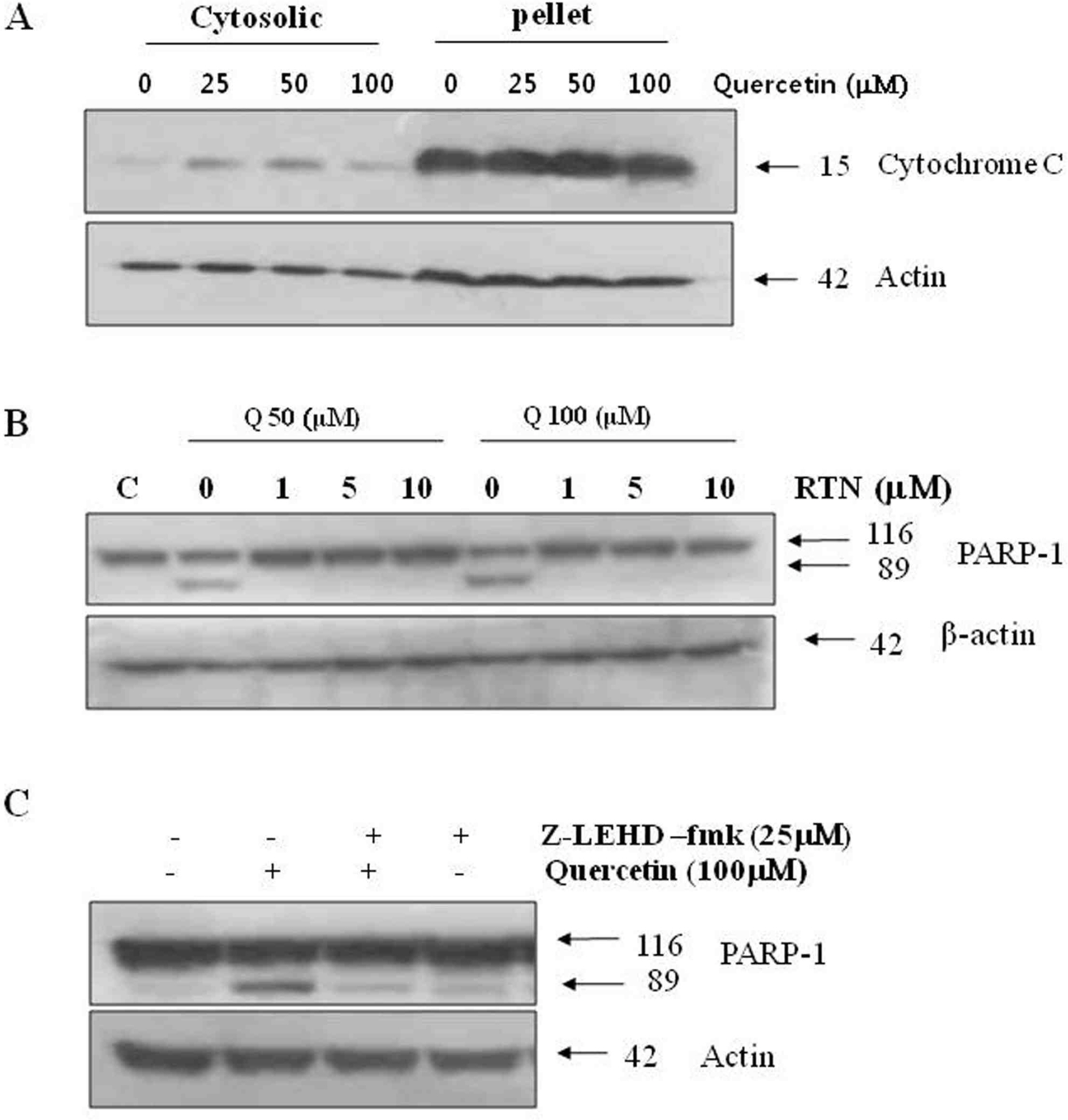
Effects of quercetin on mitochondrial dependent signaling pathway. A. PC-9 cells were treated with indicated concentrations quercetin for 24 h, while mitochondrial and cytoplasmic extracts were prepared as described in “Materials and methods” section. B. Cells were incubated with 50 uM and 100 uM quercetin for 24 h, in the absence or presence of various concentrations rotenone (RTN). C. Cell were treated with 100 uM quercetin for 24 h, in the absence or presence of caspase-9 inhibitor (z-LEHD-fmk, 25 uM). Q: quercetin.