A Case of Peritoneal Sarcomatoid Mesothelioma with Absence of Occupationl Exposure to Asbestos
Article information
Abstract
Sarcomatoid mesothelioma is not very common, mesothelioma is directly attributable to occupational asbestos exposure, with 90% of cases showing a history of exposure. A 66-year-old male was admitted with an abdominal pain that persisted for 3 weeks. He had no abdominal mass. Computed tomography showed soft tissue thickening in perihepatic space and nodularities in omentum and peritoneum with ascites. There was no absolute diagnosis evidence in ascites analysis. Although the pathology of ascites was free for malignancy, the patient underwent omentum biopsy for definitive diagnosis. In laproscopic exploration, there was omental cake, peritoneal nodular seeding. It was suspected cancer carcinomatosis. Immunohistochemical findings suggested that it was sarcomatoid masothelioma. This is the rare case of a peritoneal sarcomatoid mesothelioma, without any exposure to asbestos.
Sarcomatoid mesothelioma is an uncommon type of cancer. This is the most serious of all asbestos-related diseases. It only occurs in 7 to 20 percent of all mesothelioma cases. Idiopathic or spontaneous mesothelioma can also occur in the absence of any exposure to asbestos, with a spontaneous rate in humans of around one per million. We report the rare case of a peritoneal sarcomatoid mesothelioma, without any exposure to asbestos. If sarcomatoid mesothelioma cancer's symptoms are cataloged and asbestos exposure history is determined, radiologic imaging studies are to be followed. If radiologic studies show serious abnormality, the acquisition of pathology is needed. Tissue samples are analyzed and examined in the laboratory, which normally contradict or confirm the sarcomatoid mesothelioma. The sarcomatoid mesothelioma's tissues are hard to distinguish from healthy tissues in most cases, making it a challenge to accurately diagnose sarcomatoid mesothelioma.1 Unfortunately, sarcomatoid mesothelioma cancer is very resistant to treatments. As such mesothelioma has a very poor prognosis.2 The average survival of sarcomatoid mesothelioma diagnosed is about 8 months.3
CASE
A 66-year-old male was admitted with a persistent abdominal pain for 3 weeks and dyspepsia. He was a lifelong farmer, taking medication for hypertension and diabetes mellitus. He has lived in one area for lifetime, there was no asbestos-related facility. He had never been exposed asbestos, and had received appendectomy, 10 years ago. His smoking status was 20 pack-years, his family history was negative for malignancy. On admission, his vital sign was stable, and direct tenderness was noted over the right side of the abdomen, but mass like lesion was absent. The complete blood count showed a white cell count of 10,800 /mm3 (neutrophils 69.6%, lymphocytes 20.2%), hemoglobin 14.6 g/dL, and platelet count of 172,000 /mm3. The blood chemistry showed a Total bilirubin 0.7 mg/dL, ALP 67 IU/L, AST/ALT 45/36 U/L, r-GT 87 IU/L, BUN 20.9 mg/dL, Cr 1.0 mg/dL, and CRP 5.30 mg/dL. The CEA was 1.8 ng/mL, and AFP was 11.5 ng/mL. Blood, urine cultures did not grow any bacteria or fungi. CT showed soft tissue thickening in the perihepatic space and nodularities in omentum and peritoneum with ascites (Fig. 1). Further, there were multiple calcified lymph nodes in mesentery. Colonoscopy showed sigmoid colon polyp, without mass or inflammation. Histopathology of sigmoid colon polyp showed tubulovilous adenoma with low grade dysplasia. The peritoneal fluid analysis showed pH 7.0, WBC 5600 /uL (lymphocyte 54%, monocyte 35%), RBC 5250 /uL, CEA 0.7 ng/mL, CA 19-9 0.6 U/mL, ADA 28.5 IU/L, INF-gamma (-), and the peritoneal fluid was a exudate (serum-ascites albumin gradient 1.1). Peritoneal fluid cultures did not grow any bacteria or fungi. As a result of the study, we ruled out tuberculous peritonitis or peritoneal carcinomatosis. So we planned diagnostic omentum biopsy, and laproscopic exploration was done. In laproscopic exploration, there was omental cake, peritoneal nodular seeding (Fig. 2). The tumor consists of pleomorphic spindle cells arranged in fascicles or haphazard distribution with focal chronic inflammatory infiltrate. The tumor cells have oval hyperchromatic nuclei and prominent nucleoli. The tumor shows high mitotic activity (67 mitotic figures/50 high power field) and focal necrosis. In immunohistochemical stain, the tumor cells manifest positive reactivity for pancytokeratins, but negative for CD117, CD34, S-100 protein, desmin, calretinin, and CEA. According to the histologic findings and immunohistochemical results, the most likely diagnosis is sarcomatoid mesothelioma (Fig. 3). Finally, we concluded to the diagnosis of idiopathic peritoneal sarcomatoid mesothelioma. However, the patient refused management.
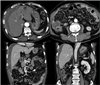
Computed tomography reveals soft tissue thickening in perihepatic space and peritoneum (thin arrows) with omental nodularities. There are small amounts of ascites in perihepatic space (thick arrows).
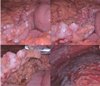
In laproscopic exploration, there was omental cake, peritoneal nodular seeding . It was suspected cancer carcinomatosis.
DISCUSSION
Before 1950, malignant mesothelioma was so rare that even its existence was questioned by some pathologists.4 However, with the progression of time, the reported cases of malignant mesothelioma increased. Although its use was widely abandoned in the western world in the 1980s, the long latency period between exposure to asbestos and onset of mesothelioma, which can range from 15 to 60 years, meant that the mortality rates from mesothelioma have continued to rise.5 Idiopathic or spontaneous mesothelioma can occur in the absence of any exposure to asbestos in both animals and humans, thus, a recent study suggests that idiopathic mesothelioma rate in humans is one per million.67 In this case, the patient was 66 years without any exposure of asbestos.
Pleural malignant mesothelioma is the most common form of mesothelioma. Typical presenting features are those of chest pain and dyspnea. Breathlessness due to a pleural effusion, without chest pain, has been reported in about 30% of patients.8 Presentation of peritoneal mesothelioma is with non-specific symptoms, which includes the loss of appetite, nausea and vomiting, diarrhea or constipation and occasionally ascites.7 Even in this case, there were only non-specific symptoms, including abdominal pain and dyspepsia.
Blood test is not helpful for mesothelioma diagnosis. There can be an elevated erythrocyte sedimentation rate.9 The combination of accurate history, examination, radiology, and pathology are essential factors in the diagnosis of mesothelioma. X-ray, CT, MRI and PET have all been used to evaluate the disease.10 Malignant mesothelioma can be classified as epithelioid, sarcomatoid, or biphasic based on tissue obtained by biopsy. The pathological diagnosis is reached with the aid of immunohistochemistry to demonstrate the presence of mesothelial, epithelial, or true sarcomatous differentiation in the malignant cell.11 There is no individual immunohistochemical mesothelial marker that provides 100% specificity and high sensitivity for the diagnosis of malignant mesothelioma.11 Markers, such as keratin, mesothelin, caliretinin, and thorombomodulin have currently been used for the diagnosis and differentiation of mesothelioma. In this case, the tumor consisted of pleomorphic spindle cells arranged in fascicles or haphazard distribution, the tumor cells manifested positive reactivity for pancytokeratins.
Unfortunately, sarcomatoid mesothelioma is resistant to treatment, which means that any treatments, including surgery, chemotherapy, and radiotherapy becomes insignificant in treating sarcomatoid mesothelioma.2 The role of surgery is still uncertain, the use of chemotherapy aims to lengthen survival, improve quality of life and provide symptomatic relief.1213 Currently, there is no single drug or combination therapy that could be considered as the standard treatment.14 Radiotherapy occasionally induces regression of disease, but there is no evidence that radiotherapy affects survival.12 However, it has been helpful in the palliation of pain.15 The response to this treatment leads to a poorer prognosis for patients diagnosed with this subtype, as well as a reduced survival time. In this case, we could not confirm treatment outcome because the patient refused management, follow-up.
Life expectancy in sarcomatoid mesothelioma is poor, with median survival varying between 7 and 9 months.3 Furthermore, the prognosis of peritoneal mesothelioma is worse than pleural mesothelioma, with a mean survival time of about 7 months.9
In Korea, sarcomatoid mesothelioma is very rare. It has a poor prognosis and short survival with any treatment. We report a peritoneal sarcomatoid mesothelioma patient who had never been exposed asbestos.
