Utility of Regular Radiological Follow-up on Early Detection of Contralateral Malignancy and Long-term Outcomes in Metachronous Bilateral Breast Cancer Patients
Article information
Abstract
Objectives
We investigated the utility of regular radiological follow-up on the early detection of contralateral breast cancer(CBC) and prognosis in patients with metachronous bilateral breast cancer.
Methods
Between 1983 and 2010, 49(2.1%) metachronous bilateral breast cancer patients were identified among a total of 2,343 cases of invasive or in situ breast carcinomas. We reviewed the patients' medical records including age, stage, duration between the first and second breast cancer diagnosis, operation method, recurrence, and breast cancer-specific survival.
Results
The mean ages at the first and second breast cancer diagnosis were 43.8 and 49.2 years, respectively. The mean duration between the first and second breast cancer diagnosis was 68.9 months (range, 7–266 months). Regular radiological follow-up with annual mammography(MMG) with or without ultrasonography was conducted in 28 patients (63.6%, Group 1), and no regular follow-up was performed in 12 patients (27.3%, Group 2). The median follow-up duration was 150 months. In a comparative analysis, Group 1 patients exhibited more stage 0 and stage 1 malignancies (82.1% vs. 25%, P =0.006) as second cancer and the same or an improved stage (71.4% vs. 33.3%, P =0.042) of second cancer compared to Group 2 patients. Breast cancer-specific survival rates between the two groups after the first cancer occurrence were higher in Group 1 patients compared to Group 2 patients, although this did not reach statistical significance.
Conclusion
Screening for CBC with regular radiological follow-up could result in early detection of CBC, less invasive surgical procedures, and enhanced breast cancer-specific survival outcomes.
Breast cancer represents the second most common type of cancer in Korean women, and its incidence is increasing annually. With advances in screening and national healthcare services, more than half of newly diagnosed breast cancers in Korea are in situ or stage 1 carcinomas.1 Early detection of the disease and a multimodal treatment approach that is standard in the care of breast cancer patients has meant that the survival rates in Korea are as high as other top-ranking countries. This change has led many breast cancer survivors to keep concerns on their breast cancer issues.
It is generally accepted that aggressive follow-up to detect distant metastatic lesions in women with a prior history of breast malignancy is not beneficial for enhancing the overall survival rates. By contrast, mammography(MMG) is the only recommended imaging modality for the surveillance of these patients.234 Conventionally, annual MMG follow-up is recommended for the surveillance of ipsilateral breast tumor recurrence and contralateral breast cancer (CBC) development after treatment for unilateral breast cancer. However, no randomized controlled trials on the benefits of regular MMG follow-up in women with a prior history of breast malignancy have been conducted. Several epidemiologists have reported that regular MMG follow-up reduces mortality rates among breast cancer survivors.56 Some studies have evaluated the diagnostic potential of screening MMG in patients with a prior history of breast malignancy and demonstrated that regular MMG follow-up could detect early-stage secondary breast cancers.78 However, limited studies have been conducted concerning the diagnostic and prognostic potential of regular MMG follow-up in metachronous bilateral breast cancer patients who developed CBC whilst under surveillance. In this study, we investigated the diagnostic potential and long-term survival outcomes of regular MMG follow-up in a cohort of metachronous bilateral breast cancer patients.
MATERIAL AND METHODS
Metachronous CBC occurred in 49 (2.1%) of 2,343 patients who underwent radical surgery for unilateral breast malignancies, including both in situ and invasive carcinomas, at our institute between 1983 and 2010. Patients who developed bilateral breast cancer within a 6-month period or direct spread to the contralateral breast were excluded, leaving a total of 44 patients comprising the final study population. These patients were stratified into two groups according to whether regular radiological follow-up was performed. The criteria for regular radiological follow-up were met provided the patients visited the hospital for MMG with or without ultrasonography at least once a year. Patients who did not visit the hospital after receiving treatment for unilateral breast malignancies and who were subsequently diagnosed with CBC by their symptoms or physical examinations were regarded as not having regular radiological follow-up. Regular radiological follow-up was routinely recommended for breast cancer survivors. Biannual clinical breast examinations were conducted during the first 5 years after surgical treatment of breast cancer, and offered on an annual basis thereafter. MMG was performed every 6 or 12 months during the first 5 years, and then once a year thereafter. MMG and ultrasonography were conducted simultaneously in women with dense breast tissue who could not be assessed by MMG alone.
We retrospectively reviewed the patients' medical records and compared a number of clinical parameters including age, stage, duration between the first and second breast cancer diagnosis, operation method, recurrence, and breast cancer-specific survival. Breast cancer-specific survival was measured from the date of first breast cancer diagnosis to the date of death or last follow-up. Pathologic staging was based on the 7th American Joint Committee on Cancer (AJCC) criteria. The significance of the differences between these two groups was determined using a chi-square test. The significance of survival was calculated using the Kaplan-Meier method, log-rank test, and Breslow test. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) for Windows Software Version 18.0(SPSS Inc., Chicago, IL, USA). This study was approved by the Institutional Review Board of our institution (DSMC201602031).
RESULTS
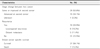
The mean ages at the first and second breast cancer diagnosis across all 44 patients were 43.8 and 49.2 years, respectively. Patients who underwent surgery for unilateral breast malignancies in the same period exhibited an older age of breast cancer diagnosis (mean 49.5 years) that was statistically significant (P = 0.001, data not shown) when compared to the mean age at the first breast cancer diagnosis in metachronous bilateral breast cancer patients. The median follow-up duration was 150 months (range, 29–336 months). The mean duration between the first and second breast cancer diagnosis was 68.9 months (range, 7–266 months). Regular radiological follow-up with an annual MMG with or without ultrasonography was conducted in 28 patients (63.6%, Group 1), and no regular follow-up was performed in 12 patients (27.3%, Group 2). For the remaining 4 patients (9.1%), medical information was not available regarding their follow-up study. At the point of diagnosis of the first breast malignancy, in situ, stage 1, stage 2, and stage 3 carcinomas were identified in 5 (11.4%), 11 (25%), 21 (47.7%), and 6 (13.6%) patients, respectively. Stage of first breast malignancy of remaining 1 patient was not available because of missing data. Conversely, at the point of diagnosis of the second breast malignancy, in situ, stage 1, stage 2, stage 3, and stage 4 carcinomas were identified in 7 (15.9%), 20 (45.5%), 11 (25.0%), 5 (11.4%), and 1 (2.3%) patients, respectively(Table 1). Second cancers tended to be detected at an earlier stage than first cancers, with 28 patients (63.6%) exhibiting the same or improved stage at the point of diagnosis of the second malignancy compared to the point of diagnosis of the first malignancy (Fig. 1). Thirteen patients demonstrated recurrences. Of these, 7 patients died (Table 2).

Distribution of stage according to first and second cancer event. Dotted line means the patients that two events of cancer exhibited the same stage. Area under the dotted line means the patients whose stage of second cancer was improved. Area over the dotted line means the patients whose stage of second cancer was advanced.
In a comparative study, differences in the mean age at diagnosis and duration between the first and second cancer diagnosis were found not to be statistically significant between the two groups. The stage at the first cancer diagnosis exhibited a similar distribution pattern between the two groups. However, the stage at the second cancer diagnosis demonstrated that early-stage (in situ or stage 1) carcinomas were more prevalent among the Group 1 than among the Group 2 patients (82.1% vs. 25.0%, P = 0.006). Additionally, more Group 1 patients detected their second malignant lesion in the contralateral breast at an same or earlier stage than they had their first malignant lesion (71.4% vs. 33.3%, P = 0.042). Owing to more advanced stage in the second breast cancer diagnosis among Group 2 patients, more mastectomies were performed (75% vs. 25%, P = 0.012). Six patients suffered breast cancer-related deaths. Of these, 3 patients (10.7%) were in Group 1, 3 patients (25%) were in Group 2 (Table 3). The 10- and 15-year breast cancer-specific survival rates among all study participants after the first breast cancer diag nosis were 94.1% and 82.7%, respectively. Comparing the 10- and 15-year breast cancer specific survival rates between Group 1 and 2, Group 1 showed better result than Group 2, however, there is not statistical significance (log rank test, 0.179, Breslow test, P = 070) (Fig.2).

Breast cancer specific survival measured from 1st cancer diagnosis were compared between Group 1 and Group 2.
We subsequently stratified the patients according to stage change between the two cancer events. The stage of the second breast malignancy was similar or improved in 28 patients (63.6%), and more advanced in 15 patients (34.1%) than the stage of the first breast malignancy, at diagnosis. Patients with more advanced second cancer presented earlier mean age of first breast malignancy (38.9 vs. 46.3 years, P = 0.034) with as few as 7 of these patients (46.7%) having received routine follow-up with MMG. In contrast, 20 patients (83.3%, P = 0.042) with similar or improved stages of secondary cancer received routine follow-up with MMG (Table 4).
DISCUSSION
The annual incidence rate of metachronous CBC has been reported to be approximately 0.3% to 0.6%.9 Recent studies have been published which suggest that the incidence rate of metachronous CBC has been declining since the 1980's. The authors attributed this trend to the increasing use of adjuvant endocrine therapies.1011 The Early Breast Cancer Trialists' Collaborative Group identified the effectiveness of tamoxifen in the prevention of CBC, specifically noting a risk reduction of almost 38% in estrogen-receptor-positive disease with approximately 5 years of adjuvant tamoxifen therapy.12 In the Arimidex, Tamoxifen, Alone or in Combination trial, 5 years of anastrozole treatment was found to be more effective than that of tamoxifen in the prevention of CBC in estrogen-receptor-positive post-menopausal women.13
Risk of developing contralateral breast cancer is high in young age and this is demonstrated in recent report using National Cancer Institute's Surveillance, Epidemiology, and End Result database.10 In our study, age of first breast cancer was younger in metachronous bilateral breast cancer patients than unilateral breast cancer patients. Estrogen receptor negative-breast cancer is another risk for developing CBC, because tamoxifen and aromatase inhibitor has contributed to decrease the incidence rates of CBC in estrogen positive-primary breast cancer. Lee et al reported that risk of developing CBC in HER2 subtype and triple negative subtype first primary breast cancer was significantly higher compared to luminal A subtype breast cancer.14 Mutation of BRCA gene is also a contributing factor for CBC, so careful surveillance on contralateral breast is mandatory in breast cancer patients with positive BRCA mutation.1516 Bilateral breast cancer is known to be associated with a higher risk of mortality than unilateral breast cancer, with poorer outcomes recorded in a subgroup of patients that exhibited younger-onset and shorter durations between the first and second cancer diagnosis.11171819
Several studies have reported mean durations between the occurrence of the primary breast cancer and CBC as being > 5 years, which means patients need to pay careful attention to their contralateral breast for a significant amount of time after receiving treatment for the primary breast cancer.1720 In these patients, an annual MMG and physical examination are recommended as routine practice. Because the detection of early breast cancer has increased worldwide and adjuvant therapies have improved survival outcomes, the health concerns of long-term survivors have become an important issue, including the development of CBC. However perceptions for their CBC development are getting attenuated over time after treatment of breast cancer, so physicians should inform patients about the risks of CBC and maintain their surveillance.21
Young-onset breast cancer is well known to be associated with a poor prognostic outcome. In our study, metachronous CBC usually occurred in women who had received a diagnosis of breast cancer at an early age. Moreover, advanced stage breast cancer was diagnosed more frequently at the second event than at the first event in young-onset patients. A review published by Houssami and Ciatto also reported that CBC in young-onset patients was more likely to be diagnosed in advanced disease and detected through the presentation of symptoms.9 Routine MMG follow-up could detect early-stage CBC, although this is not applicable in young-onset breast cancer patients. However, surveillance guidelines do not offer specific recommendations according to different age groups; therefore, there are limitations in the management of young-onset breast cancer patients.
In recent decades, bilateral mastectomy for the surgical treatment of unilateral breast cancer has been increasingly performed in the United States despite the decline in incidence rates of CBC development.22 This procedure is used for the purpose of surgical treatment of unilateral breast cancer, and the prophylactic procedure of CBC development. Several studies have presented confounding results on the proposed benefits of bilateral mastectomy for sporadic unilateral breast cancer.232425 However, two meta-analyses have recently concluded that routine use of contralateral prophylactic mastectomy in sporadic breast cancer patients did not have a significant risk reduction on survival.2627 It has not been well established who is likely to benefit most from contralateral prophylactic mastectomy.28 In this study, the 10- and 15-year breast cancer-specific survival rate in stage 0 to stage 2 metachronous bilateral breast cancer patients was 100% and 82.1%. Of these, stage 0 to stage 2 metachronous bilateral breast cancer patients, one had a BRCA1 mutation and another had a family history of multiple breast cancers, but both survived to last follow-up without recurrence. This means that adequate control of disease progression is possible with screening, surveillance programs, and proper treatment, without the need for performing the most invasive surgical procedures like prophylactic bilateral mastectomy or contralateral prophylactic mastectomy.
This study has several limitations, including its retrospective design. It is also a single center study with a relatively small cohort, owing to the fact that the incidence rates of CBC are exceedingly low and the durations between the two cancer events are long. Nationwide studies can derive more precise and high-level prognostic value on regular MMG follow-up.
In conclusion, CBC represents the most common second primary cancer among breast cancer survivors. Routine screening of the contralateral breast could enhance the early detection of CBC, possibility of breast conserving surgery, and enhanced breast cancer-specific survival outcomes.
ACKNOWLEDGEMENT
The authors declare that they have no competing interests.