Predominant Myofibrillar Pathology with Preserved Sarcolemmal Aquaporin 4 Immunoreactivity in a Patient with Neuromyelitis Optica-Associated HyperCKemia
Article information
Abstract
A 49-year-old man developed recurrent myalgia and hyperCKemia during acute attacks of neuromyelitis optica. Muscle biopsy was performed, and the pathological findings were analyzed. Predominant myofibrillar pathology was observed, which constitutes a unique finding that has not been reported before. This case result shows that neuromyelitis optica-associated hyperCKemia can produce variable pathologic phenotypes. Further studies are needed to elucidate the relationship between myofibril destruction and aquaporin 4 autoimmunity.
Neuromyelitis optica (NMO) and NMO spectrum disorder (NMOSD) represent a continuum of inflammatory autoimmune diseases of the central nervous system (CNS) that are classically restricted to the optic nerves, spinal cord, and brain.1 The NMO-immunoglobulin G (IgG) antibody is a specific serum autoantibody biomarker of NMOSD that targets the water channel protein aquaporin 4 (AQP4) in astrocytes. Although AQP4 is expressed in various tissues, including skeletal muscle, kidney, stomach, and placenta, the clinical manifestation of NMO is nearly always restricted to the CNS.2–4
Several recent reports have described patients with NMO who developed high serum creatine kinase (CK) levels (hyperCKemia) during NMO attacks.5–9 Histochemical analyses of the skeletal muscle in these patients showed a variable loss of immunoreactivity against AQP4, as well as deposition of IgG and complements at the sarcolemma, suggesting that NMO IgG antibodies may contribute to the development of sarcolemmal injury and hyperCKemia. In this report, we describe a 49-year-old man with NMO who developed unexplained hyperCK-emia during NMO attacks, in whom the muscle biopsy showed destruction of myofibrils without loss of sarcolemmal AQP4.
CASE
A previously healthy 49-year-old man presented to another hospital with myalgia, urinary retention, and progressive paresthesia for 2 weeks. He was also noted to have unexplained high levels of serum CK (2,386 IU/L; normal range: 49–397 IU/L). Magnetic resonance imaging (MRI) of the spine demonstrated longitudinal extensive transverse myelitis extending from C7 to T4. The patient was treated with 1 g of intravenous methylprednisolone (IVMP) for 3 days. Five days after cessation of IVMP, he developed reduced visual acuity bilaterally (OD, 0.1; OS, 0.04) and MRI of the orbit demonstrated enhancement of the bilateral optic nerves. He visited our hospital for a further evaluation 1 week after the second cycle of IVMP. After admission to our center, he developed worsening visual acuity in the right eye (OD, 0.025; OS, 0.1), recurrent hiccups, and quadriplegia. Follow-up spinal cord MRI showed new lesions in the medulla that were continuous to the cervical cord, and the serum anti-AQP4 antibody test yielded positive results. The results of serological testing were negative for antineutrophil cytoplasmic antibodies, rheumatoid factor, and antinuclear antibody.
Based on these findings, the patient was diagnosed with NMO. He was also noted to have an unexplained persistent elevation of serum CK levels (> 3,000 IU/L). After seven cycles of plasma exchange followed by mycophenolate mofetil with prednisolone, the patient started to show progressive improvement, with normalization of his serum CK levels. Three weeks after plasma exchange, the patient began to show a gradual rise of serum CK levels to 1,458 IU/L with myalgia. Needle electromyography showed profuse fibrillation potentials and positive sharp waves with myopathic motor unit potentials in the bilateral proximal limb muscles, suggesting active myopathy. In order to investigate the cause of hyperCKemia, a muscle biopsy was performed in the biceps. Two weeks after muscle biopsy, the patient rapidly developed altered mentality and left hemiparesis. Serum CK levels had further increased (> 3,000 IU/L). Brain MRI revealed typical NMO lesions involving the genu of the corpus collosum and the corticospinal tract of the right midbrain. Muscle biopsy revealed disruption of intermyofibrillar networks without inflammatory cellular infiltrates or necrotic/regenerating fibers on light microscopic observations. Electron microscopy revealed disruption of myofibrils commencing at the Z-disk, loss of myofilament density, and scattered electron-dense materials. Immunohistochemistry showed upregulated human IgG-Fc, C5b-9 antibody, and major histocompatibility complex (MHC) class I antigen, but normal AQP4 expression at the sarcolemma (Fig. 1). Myofibrillar myopathy-related mutations were not identified in whole-exome sequencing. After five cycles of plasma exchange and subsequent rituximab treatment, the patient showed gradual neurological improvement with normalization of serum CK levels. During the 20 months of follow-up, the patient had no further relapse.
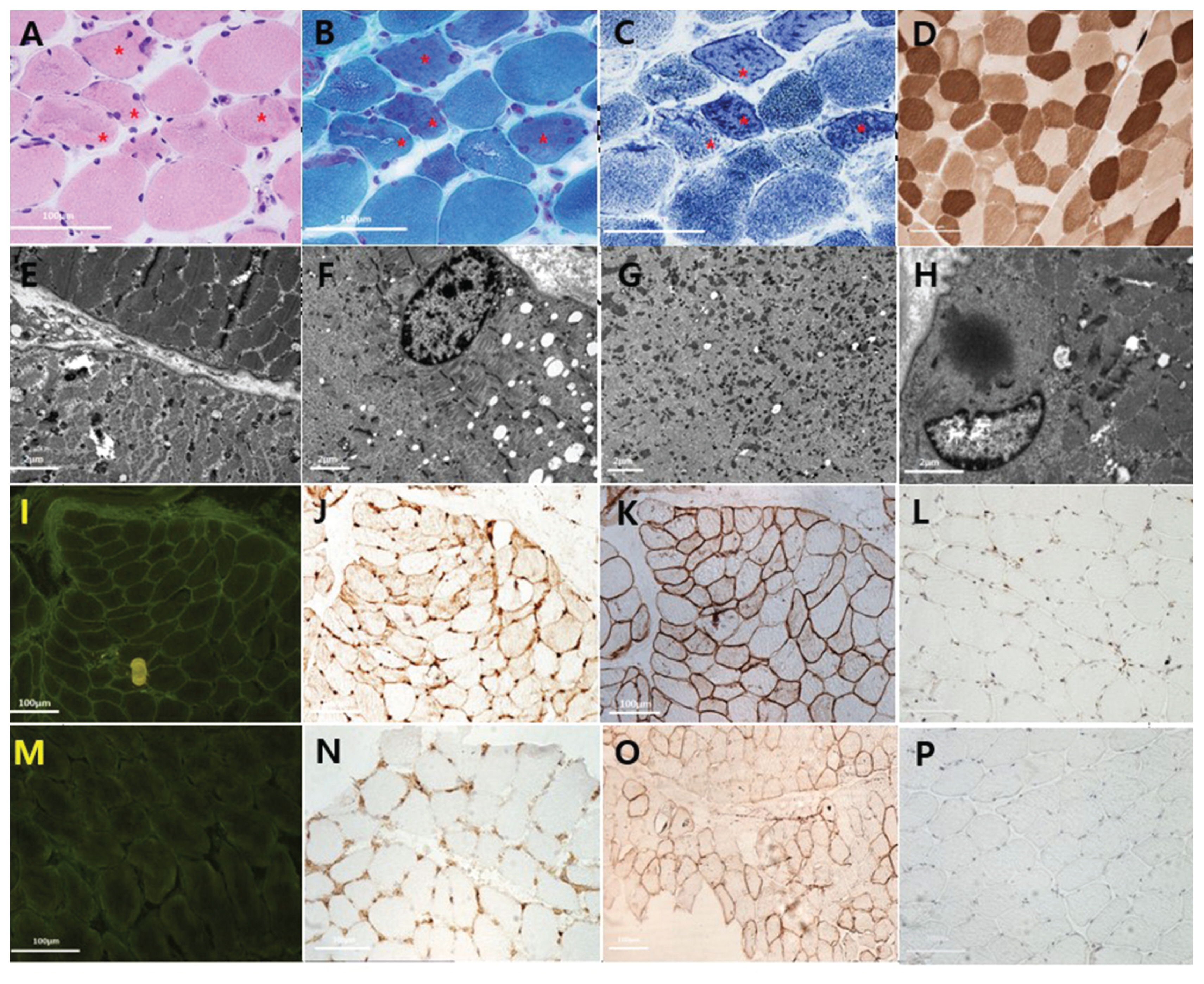
The patient’s muscle biopsy (A–L) and a normal control for comparison (M–P).
Light microscopy findings of hematoxylin-eosin staining (A), modified Gomori-trichrome staining (B), nicotine amide dehydrogenase staining (C), and adenosine triphosphatase staining at pH 4.6 (D) demonstrate variability in fiber size and irregular sarcoplasmic structures (asterisks). Electron microscopic findings show destruction of myofibrillar structures, with loss of myofilament density on the left lower side (E), disorganized Z-disk alignment except for the perinuclear area (F), numerous scattered electron-dense materials (G), and cytoplasmic body formation (H). The immunohistochemical findings include increased immunoreactivity against human IgG-Fc (I), major histocompatibility complex class I (J), and C5b-9 antibody (L), but sarcolemmal AQP4 is well preserved (K) in the patient compared with a normal control (M–P).
DISCUSSION
In the present study, we describe a case of acute myopathy involving hyperCKemia that was associated with an NMO attack. NMO and NMOSD represent a continuum of inflammatory autoimmune diseases of the CNS.1 However, several cases of transient hyperCKemia with NMO have been reported since 2010.5–9 In these patients, the predominant myopathic symptoms were muscle pain and fatigue. The majority of individuals were asymptomatic or experienced mild proximal weakness. Although their symptoms were mild, serum CK levels were markedly increased (12,560 – 147,000 U/L) in most cases.5–9 The case presented herein also showed myalgia and hyper-CKemia without definite muscle weakness preceding or at the time of the NMO attacks; this pattern is consistent with previous reports.
Histopathologically, the predominant findings of the CNS lesions of acute NMO are extensive demyelination, substantial axonal damage, and inflammatory infiltrates, including neutrophil and eosinophil granulocytes.10 In contrast, muscle pathology findings in patients with NMO-associated hyperCKemia are generally unremarkable, without overt inflammatory reactions, except for one reported case that showed endomysial and perivascular inflammatory infiltrates.9 In other reported cases, the pathological changes were minimal, including mild fiber size variation and even normal findings.7,8 Therefore, it was suggested that minimal muscle histopathologic findings are a feature of NMO-associated hyperCKemia.8 Interestingly, the main pathological feature of the presented case was disruption of the myofibrillar architecture without any evidence of overt sarcolemmal damage or inflammatory reactions. To our knowledge, predominant myofibrillar pathology without overt muscle fiber damage has not previously been reported in a patient with NMO-associated hyperCKemia.
Previous immunohistochemical studies in patients with NMO-associated hyperCKemia showed a loss of AQP4 immunoreactivity and infiltration of IgG and complements on the sarcolemmal membrane, suggesting that AQP4 autoantibodies play a pathogenic role in the development of hyperCKemia.8,9 The present case also showed deposition of human IgG-Fc and complements, as well as elevated MHC class I expression. These findings strongly suggest that the hyperCKemia observed in the presented case was caused by an autoimmune reaction.11 The predominant myofibrillar pathology observed in this case is also of interest. Acute myofibrillar damage is frequently observed in necrotic and regenerating fibers, both of which are frequent features in inflammatory myopathies and rhabdomyolysis. In light of the otherwise unremarkable muscle biopsy findings with no necrotic or regenerating fibers in this patient, as well as his relatively short clinical course, the myofibrillar destruction observed in the case was unlikely to have been caused by muscle fiber necrosis or regeneration. Thus, unidentified autoantibodies produced during the flare-up of an NMO attack might have led to myofibrillar destruction observed in the muscle biopsy of this case. Another possibility is that preserved AQP4 immunoreactivity might have been related to the timing of muscle biopsy and disease activity. Since the muscle biopsy was performed after plasmapheresis with immunotherapy and prior to the devastating attack of NMO in our case, AQP4 molecules in the sarcolemmal membrane might have still been preserved from the severe antibody-mediated destruction. A previous case report described a normal presentation of sarcolemmal AQP4 in a patient with NMO despite recurrent hyperCKemia.7 Based on these findings, hyperCKemia in a patient with NMO may be associated with varying degrees of AQP4 immunoreactivity and a variety of phenotypes on pathologic findings. However, we could not find clear evidence for the role of the AQP4 autoantibody in myofibril destruction. In order to elucidate the exact mechanism of NMO-associated hyperCKemia, it will be necessary to analyze more cases with well-designed pathological studies.