Removal of Large Urinary Stone Using Percutaneous Nephrolithotomy in a Patient with Crohn’s Disease
Article information
Abstract
Extraintestinal manifestation (EIM) of inflammatory bowel disease (IBD) is approximately 36%. Of genitourinary complications as an EIM of Crohn’s disease (CD), nephrolithiasis is the most common urinary complication in patients with CD. CD patients have been shown to have decreased urinary volume, pH, magnesium, and excretion of citrate, all of which are significant risk factors for nephrolithiasis. Genitourinary complications often occur in case of a severe longstanding disease and are associated with, the activity of bowel disease, especially in those who have undergone bowel surgery. As uncontrolled nephrolithiasis could impair renal function as well as adversely affect quality of life, proper monitoring, early detection, and prevention of the occurrence of urologic complications in CD is crucial. Few data are available about urolithiasis in patients with CD. Herein we report a case of a successful removal of a 2.7 cm calcium oxalate stone using percutaneous nephrolithotomy from a patient with long-standing CD with a previous surgery for small intestinal and colonic stricture.
Extraintestinal manifestation (EIM) of inflammatory bowel disease (IBD) is approximately 36%.1 IBD consists predominantly of ulcerative colitis (UC) and Crohn’s disease (CD). EIMs may show dermatological, musculoskeletal, ocular, vascular, genitourinary, cardiac, pulmonary, hepatobiliary, hematological, endocrine, or metabolic involvement.2 A multidisciplinary team approach is often needed for effective management, and emergency situations require prompt evaluation.
Genitourinary complications as an EIM of CD include nephrolithiasis, non-calculous urinary obstruction, tubulointerstitial nephritis, glomerulonephritis, enterovesical fistula, and amyloidosis.3 Genitourinary complications often occur in case of a severe long-standing disease and are associated with, the activity of bowel disease, especially in those who have undergone bowel surgery.4 Nephrolithiasis is the most common urinary complication in patients with CD.3 Patients with CD are more likely to develop nephrolithiasis than the general population whose lifetime nephrolithiasis prevalence is 5–10%. Nephrolithiasis is reported in 1.5–16% of patients with CD, with a prevalence of 1.5–5% for patients who have not undergone bowel surgery, and 3.7–16% for those who had bowel surgery. 5 This is much higher than in the general population, where the reported incidence is 0.09% of all patients admitted to hospital. A calcium oxalate stone is the most common type of stone observed.3 We report a case of a large renal stone that was successfully treated with percutaneous nephrolithotomy (PNL) in a patient with long-standing CD with a previous surgery for small intestinal and colonic stricture.
CASE
A 50-year-old female visited our clinic with watery diarrhea (> 10 times a day), abdominal pain, and intermittent hematuria for 3 to 4 months. She underwent ileal resection 25 years ago because of ileal stricture and abscess due to CD. However, the patient had not received treatment for the past 10 years. She used NSAIDs from local clinics for abdominal pain, and recently, the abdominal pain has become worse.
Her blood pressure was 120/60 mmHg, pulse 80/min, respiratory rate 20/min and body temperature 36.7 °C. Physical examination showed abdominal tenderness and Costovertebral angle tenderness; however, no rebound tenderness was noted. White blood cell count was 6.31 × 103/uL, hemoglobin 9.2 g/dL, and platelet 412 × 103/μL. The liver function test result was within the normal range, and the renal function test revealed a blood urea nitrogen (BUN) level of 12.1 mg/dL and creatinine level of 0.48 mg/dL. C-reactive protein and erythrocyte sedimentation rate increased to 3.02 mg/dL and 37 mm/h, respectively. Urinalysis showed specific gravity of 1.025 and pH of 6.0, which indicated that patient was dehydrated and the urine was concentrated and acidic. No sign of urinary tract infection was observed. However, microscopic red blood cells were found (7–10/high power field). A 24-h urine collection showed hyperoxaluria (145 mg/day), low calcium (42 mg/day), pH 5.8 and hypocitraturia (167 mg/day).
Abdominal X-ray revealed a 2.7 cm radio-opaque stone on the right upper quadrant. Abdominal computed tomography (CT) revealed right pelvicalyceal stone (Fig. 1). Stenosis of the ileocecal valve was detected during colonoscopy; thus, histologic examination was performed to detect stenosis due to malignancy. No definite evidence of dysplasia or adenocarcinoma in the biopsy specimens was found. Moreover, balloon dilatation using CRE™ balloon dilator (Boston Scientific, Boston, MA) was performed.
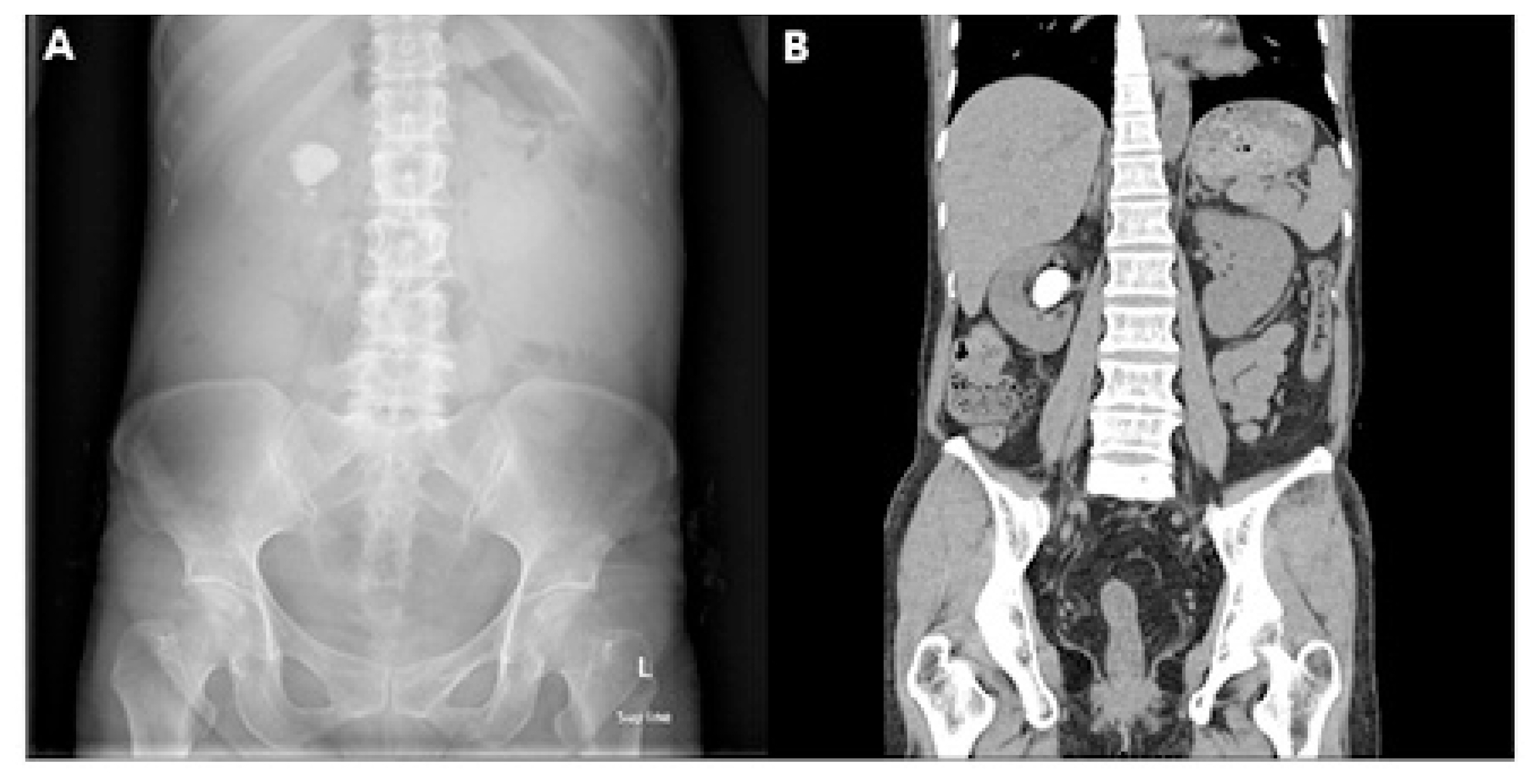
Radiologic findings at the first visit. (A) X-ray of the abdomen at the first visit showing a 2.7 cm radio-opaque stone on the right upper quadrant. (B) Computed tomography of abdomen at the first visit showing right pelvicalyceal stone.
With a general impression of moderate to severe active CD, we administered mesalazine (2g/day) and intravenous steroid (40 mg/day) for 10 days to the patient, and tapering was performed every 2 weeks after discharge. We planned a PNL for the right pelvicalyceal stone after clinical remission of CD. After 3 months from the first visit to our clinic, we performed PNL (Fig. 2). We analyzed the stone removed with PNL; the major component was calcium oxalate (Fig. 3). One month after surgery, the D-J catheter that was placed in the right ureter during PNL was removed. Abdominal CT was performed at 2 months, 1 year after surgery. The patient is currently undergoing outpatient treatment. Azathiopurine (started at 25 mg/day and maintained to 75 mg/day) to the regular maintenance regimen of infliximab (5 mg/kg every 8 weeks) and 5-ASA (2 g/day) successfully controlled the disease activity. The patient is doing well, has no abnormal findings, has no nephrolithiasis recurrence, and has been followed up in an outpatient clinic for 2 years.

Intra-operation findings of the stone. (A) Intra-operation finding of the stone. (B) Stone removal through percutaneous nephrotomy using forcep.
DISCUSSION
The pathogenesis of IBD has not been clearly established. However, chronic intestinal inflammation could lead to intestinal symptoms and complications, such as stenosis, fistula, and abscess, which in turn could result in EIMs. IBD symptoms include those due to inflammation of the gastrointestinal tract (eg, diarrhea, abdominal pain, hematochezia), generalized symptoms (eg, fever, anorexia, weight loss), symptoms of complications (eg, stricture, fistula, abscess, perforation), and EIMs. The incidence of EIMs in IBD in previous studies ranged from 21 to 41%, depending on the patient population and criteria used. EIMs were more common in CD than in UC, with a prevalence varying from 6 to 46%.6 The clinical spectrum of EIMs varied from mild transitory to very severe lesions. EIMs are varied and the diagnostic process is complex; thus, careful attention is necessary to avoid delay in the diagnosis of EIMs. Moreover, the etiology of EIMs remains unclear; hence, diagnosis is mainly based on clinical manifestations and EIM treatment is often performed empirically.7
A significant number of patients with CD may develop complications involving the kidney and genitourinary tract. The pathologic effect of CD on the genitourinary tract is primarily associated with two main clinical manifestations: inflammatory processes and metabolic abnormalities. Inflammatory processes may lead to fistula formation, cystitis, perivesical abscess and ureteral obstruction; metabolic abnormalities may be related to intestinal malabsorption that predisposes patients to nephrolithiasis. These complications may occur at any stage of CD but are usually present in a severe and long-standing disease. Early diagnosis and appropriate treatment are essential to prevent serious complications, such as renal failure.8 The relationship between IBD and nephrolithiasis was first described in 1968 by Gelzayd et al.9 Nephrolithiasis prevalence in patients with CD varies but is higher than that in the general population. Moreover, nephrolithiasis prevalence in CD is 4–5.5% (preoperative) and 15.0–30.5% (postoperative). These varied prevalence rates could be attributed to the extent of bowel disease, disease severity, and previous bowel surgery.4 The risk factors for nephrolithiasis in IBD patients are as follows: chronic dehydration, reduced physical activity, increased disease duration, inflammatory disease activity, NSAID intake, metabolic derangements, primary malabsorption, and malabsorption secondary to bowel resection.3 In patients who have undergone operations, such as small bowel resection, intestinal bypass, or total colectomy with ileostomy, the risk of nephrolithiasis is increased. A direct relationship between the development of nephrolithiasis and the amount of resected bowel exists.10 The patient in this case had all of the aforementioned risk factors, and we assumed that large renal stones developed.
In patients with IBD, nephrolithiasis is caused by urine supersaturation of components such as oxalate and urate. Therefore, low urine volume, low urine pH, low urine citrate or magnesium concentration, and high urine oxalate are risk factors for nephrolithiasis.11 Moreover, enteric hyperoxaluria (EHO) is the state of excessive urinary excretion of oxalate (> 48 mg/24 h). Increased intestinal absorption of oxalate is related to EHO, and this condition consequently increases urinary oxalate excretion, thereby inducing the formation of calcium oxalate stones. The mechanisms in EHO include the following: First, bile salt is malabsorbed in the diseased or resected distal ileum. In normal individuals, oxalate is almost exclusively absorbed by binding to calcium. However, in patients with IBD, bile salt malabsorption induced fat malabsorption (secondary hyperoxaluria) and consequently reduces the amount of calcium bound to oxalate, thereby increasing oxalate absorption.5 Second, permeability of the colonic epithelium to oxalate is increased, which is associated with secondary EHO; this condition is related to the malabsorbed bile salt and fatty acid that results from a change in the colonic epithelial tight junction due to reduced intraluminal calcium. In addition, the amount of resected ileum is related to the excretion of renal oxalate, and secondary EHO is uncommon with ileal resection of < 30 cm.12 Third, intestinal oxalate catabolism is decreased because of gastrointestinal decolonization of Oxalobacter formigenes. Extensive small bowel resection increases steatorrhea and the risk of hyperoxaluria by up to 28% because the procedure induce the disappearance of Oxalobacter formigenes or the chelation of free calcium by lipids in the colonic lumen.13 The patient in this case, had a history of ileal resection of > 30 cm, and had low urine volume, high urine specific gravity, and low urine pH, which indicated dehydration and resulted in EHO. Hence, calcium oxalate stone was formed.
Nephrolithiasis in patients with CD can be treated adequately with minimally invasive procedures, such as extracorporeal shock-wave lithotripsy (ESWL), ureteroscopy, and percutaneous surgery. ESWL is useful for renal calculi of < 2 cm in diameter and upper ureteral calculi of < 1 cm in diameter. Treatment of renal calculi with a diameter of ≤ 2 cm with ESWL results in a stone-free rate of up to 90%; however, with a lower pole position, the stone clearance rate decreases by 50%.14 Calcium oxalate stones are resistant to extracorporeal fragmentation compared with uric acid stones. Endoscopy can be used if ESWL is not successful or inappropriate because of the location, component, or size of the stone. PNL is used for large renal stones, such as staghorn calculi, and ureteroscopy is used for distal ureteral calculi.8 In the present case, ESWL was not indicated because the size of the stone was > 2 cm and the stone was located in the upper ureter. Thus, we decided to perform PNL and have successfully removed the stones. We analyzed stones and found that calcium oxalate was the major constituent. Calcium oxalate stones can be prevented by correcting EHO through, increased water intake and low fat and oxalate diet. Foods high in oxalate are green leafy vegetables and caffeinated beverages. Calcium supplements may be useful for lowering urinary oxalate. Cholestyramine, which binds bile salts in the gut, should be used only in patients without significant steatorrhea after conservative measures have failed. Pyridoxine, which reduces oxalate synthesis, could also be considered as a treatment. When calcium oxalate stones recur, urine alkalization and citrate and magnesium supplementation can be considered; however, clinical studies have not yet confirmed the efficacy of this therapy.8
Numerous patients with IBD (4–35%) have renal or genitourinary complications; thus, establishing a strategy to monitor, early diagnose, and prevent the worsening of renal or genitourinary complications is essential. However, current guidelines do not recommend regular surveillance or any specific preventive measures for nephrolithiasis. Currently, monitoring of renal function and proper clinical management has not been reported to improve the prognosis of patients with IBD, and no accurate guidance on monitoring renal function has been established. Corica and Romano suggested that monitoring renal function periodically, which includes assessment for azotemia and evaluation of creatinine levels and glomerular filtration rate, is helpful in patients with IBD.6 Specific gravity and urine pH are not only indicative of dehydration but are also risk factors for nephrolithiasis. Regular urinalysis, including specific gravity and pH; and serum BUN / creatinine measurements are extremely vital for the early detection and treatment of renal or urinary complications in IBD. This strategy should be more intensive especially for patients with previous kidney or urinary disease; those who have chronic dehydration, reduced physical activity, increased disease duration, inflammatory disease activity, NSAID intake, metabolic derangements, primary malabsorption, and malabsorption secondary to bowel resection; or those receiving potentially nephrotoxic drugs.15
Herein we report a case of a successful removal of a 2.7 cm calcium oxalate stone using PNL from a patient with a history of a small bowel surgery and a history of regular NSAID use. As uncontrolled nephrolithiasis could impair renal function as well as adversely affect quality of life, proper monitoring, early detection, and prevention of the occurrence of urologic complications in CD is crucial.
ACKNOWLEDGEMENTS
This work was supported by a 2-Year Research Grant of Pusan National University.