Efficacy of Hepatic Arterial Infusion Chemotherapy and Radiofrequency Ablation against Hepatocellular Carcinoma Refractory to Transarterial Chemoembolization and Vascular Variation: A Case Study
Article information
Abstract
Transarterial chemoembolization is often the first-line treatment for multiple hepatocellular carcinomas. However, hepatic arterial infusion chemotherapy is a treatment option for hepatocellular carcinoma refractory to multiple sessions of transarterial chemoembolization. Hepatic arterial infusion chemotherapy requires implantation of an appropriate port into the hepatic artery. However, it may be impossible to implant a port due to hepatic artery variation. We report a case of hepatocellular carcinoma refractory to transarterial chemoembolization and hepatic artery variation treated successfully with hepatic arterial infusion chemotherapy and radiofrequency ablation with complete response after implantation of ports in both liver lobes.
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related death worldwide.1 The treatment options for HCC depend on the tumor stage and performance.2 In particular, based on the Barcelona Clinic Liver Cancer (BCLC) staging system, transarterial chemoembolization (TACE) is preferred in patients with stage B carcinoma.3
However, TACE is a non-curative therapy and repeated procedures are frequently needed for disease control. Disease progression even after multiple attempts of TACE suggests refractory cancer.4 When progressing to TACE refractoriness, it is necessary to switch to other treatment options such as systemic therapy and radiation, and hepatic arterial infusion chemotherapy (HAIC) is an option if lesions are confined to the liver.5
We report a case of HCC refractory to TACE and hepatic artery variation showing sustained complete response with HAIC and radiofrequency ablation (RFA) after port implantation in both liver lobes.
CASE
A 43-year-old man visited the hospital in September 2019 seeking treatment for recurrent HCC. The patient was first diagnosed with S5/8 HCC lesion (17 mm) at another hospital in February 2018, and treated via segmentectomy, and TACE twice in January and March 2019 due to intrahepatic recurrence. In July 2019, multiple recurrences were observed in both lobes on follow-up liver dynamic magnetic resonance imaging (MRI). The patient visited our hospital to seek a second opinion. He has been taking tenofovir since January 2018 for chronic hepatitis B and did not have any other disease. His initial vital signs were stable with blood pressure 110/60 mmHg, heart rate 69 beats/minute, respiration rate 18 beats/minute, and body temperature 36.9°C. Physical examination revealed no associated symptoms or remarkable findings. The initial laboratory findings were: white blood cell count, 4,900 /μL; hemoglobin, 15.3 g/dL; platelet count, 180,000 /μL; total bilirubin, 0.8 mg/ dL; albumin, 4.4 g/dL; prothrombin timeinternational normalized ratio, 1.10; aspartate aminotransferase, 24 U/L; and alanine aminotransferase, 30 U/L. The level of alpha-fetoprotein (AFP) was 1.3 ng/mL, and protein induced by the absence of vitamin K or antagonist-II (PIVKA-II) level was 137 mAU/mL. Liver function was preserved with a Child-Pugh score of 5 and the Eastern Cooperative Oncology Group performance status was 0.
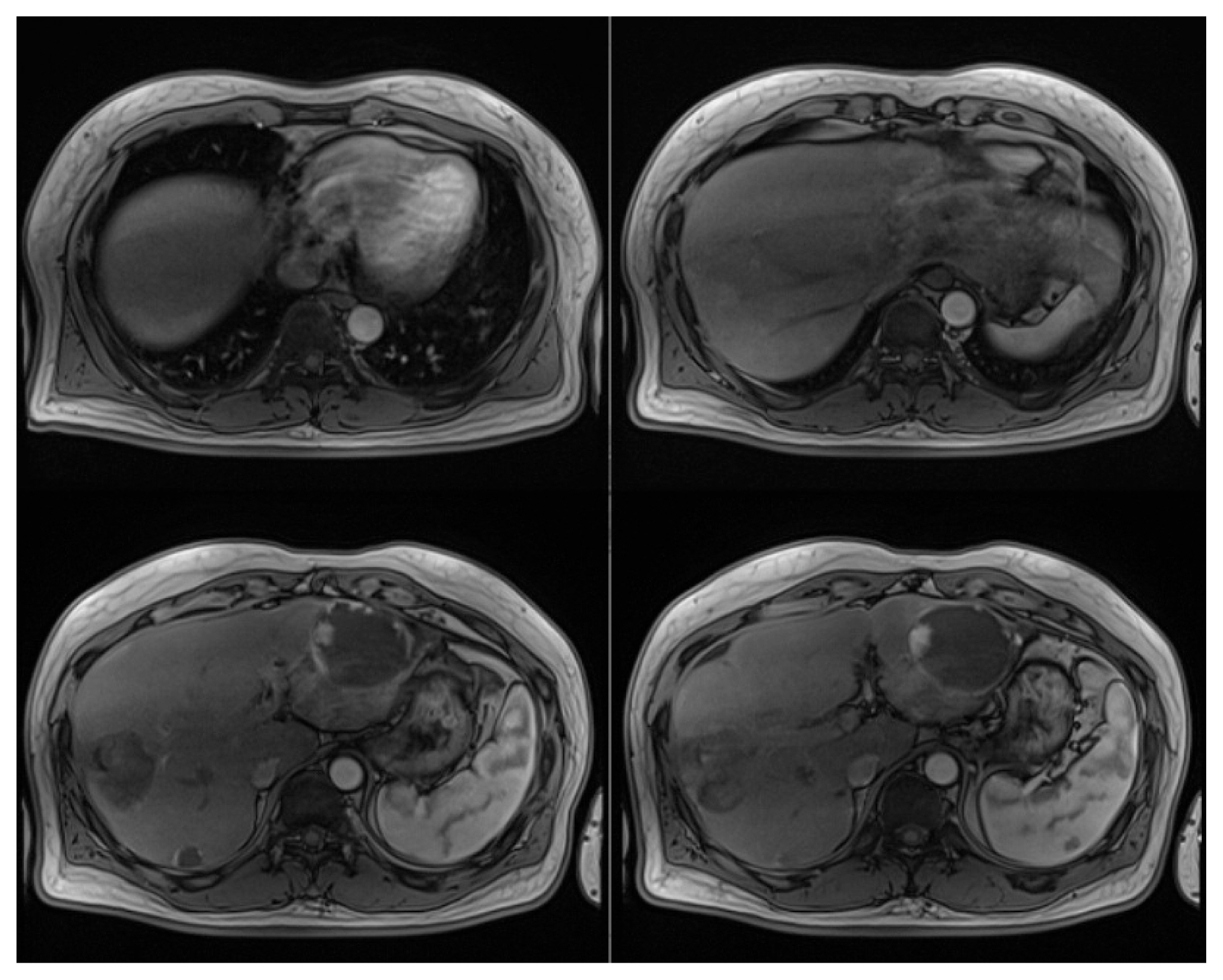
In July 2019, liver dynamic MRI revealed multiple nodules in both hepatic lobes, which are typical radiological features of HCC, arterial phase hyperenhancement and washout during the portal, delayed, and hepatobiliary phase (Fig. 1).
Hepatic dynamic magnetic resonance imaging findings (July 2019). Multiple nodules in both hepatic lobes show enhancement in the arterial phase (A), and washout in hepatobiliary phase (B). Three benign hepatic hemangiomas were homogenous with high-intensity signals on T2-weighted image (C).
The patient was diagnosed with recurrent HCC refractory to TACE based on an insufficient response after two consecutive TACE procedures and the appearance of a higher number of hepatic lesions than in the previous TACE procedure. We planned HAIC, which required implantation of a hepatic arterial port. However, the patient had vascular variation in that the right hepatic artery branched directly through the celiac trunk and the left hepatic artery branched through the common hepatic artery. To perform HAIC under vascular variation, we decided to implant two ports in each hepatic artery: one into the right hepatic artery through the right femoral artery and the other port into the left hepatic artery through the left femoral artery (Fig. 2).
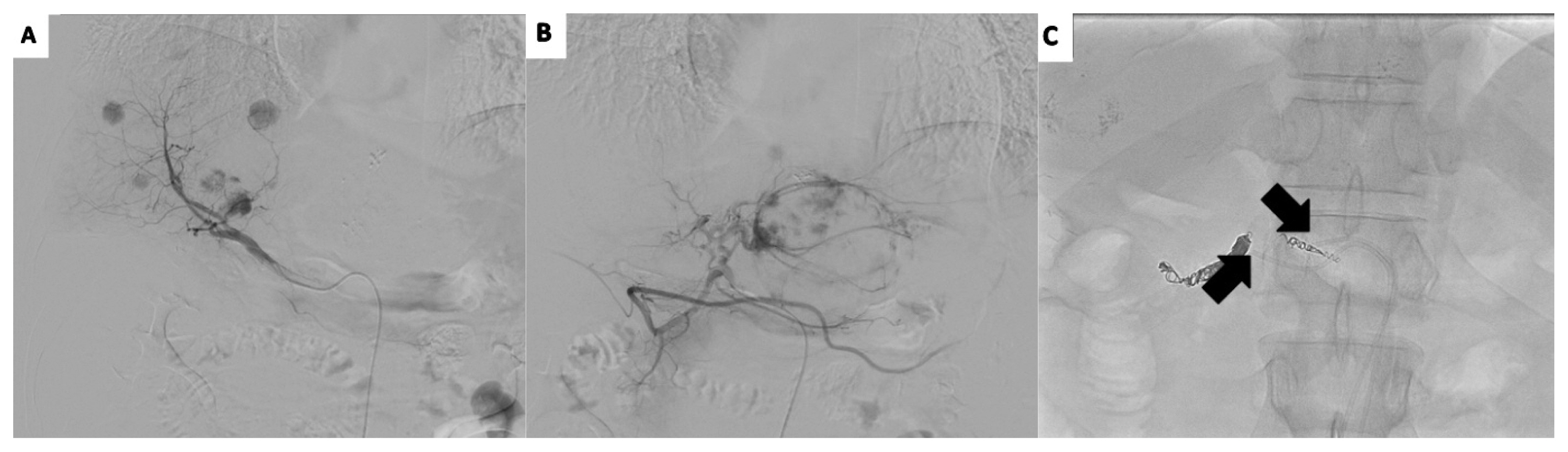
Patient’s vascular variation indicates direct branching of the right hepatic artery through the celiac trunk (A) and the left hepatic artery branching through the common hepatic artery (B). We implanted a port into the right hepatic artery through the right femoral artery and another port into the left hepatic artery through the left femoral artery (C).
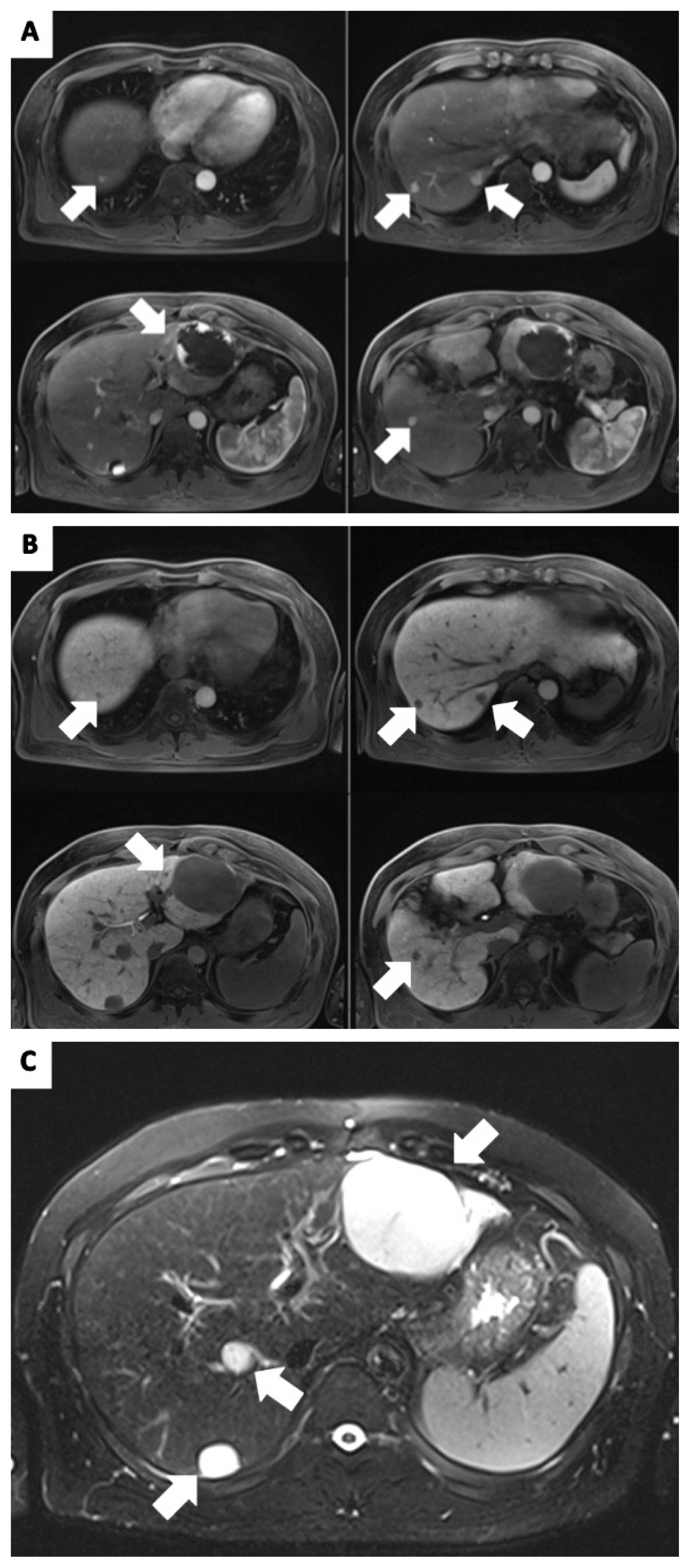
Following successful implantation of ports, we performed HAIC using Floxuridine (FUDR) chemotherapy. The patients received FUDR (0.3 mg/kg per day) divided into both ports for 14 days, followed by withdrawal for 14 days, representing a single cycle. After 3 cycles, a follow-up liver dynamic MRI was performed, which revealed multiple recurrent HCC lesions. The S5 lesion increased in size from 10 mm to 15 mm, and no other masses were detected (Fig. 3).
A follow-up liver dynamic magnetic resonance imaging in January 2020, revealed increase in the size of S5 lesion from 10 mm to 15 mm, and no other masses were observed.
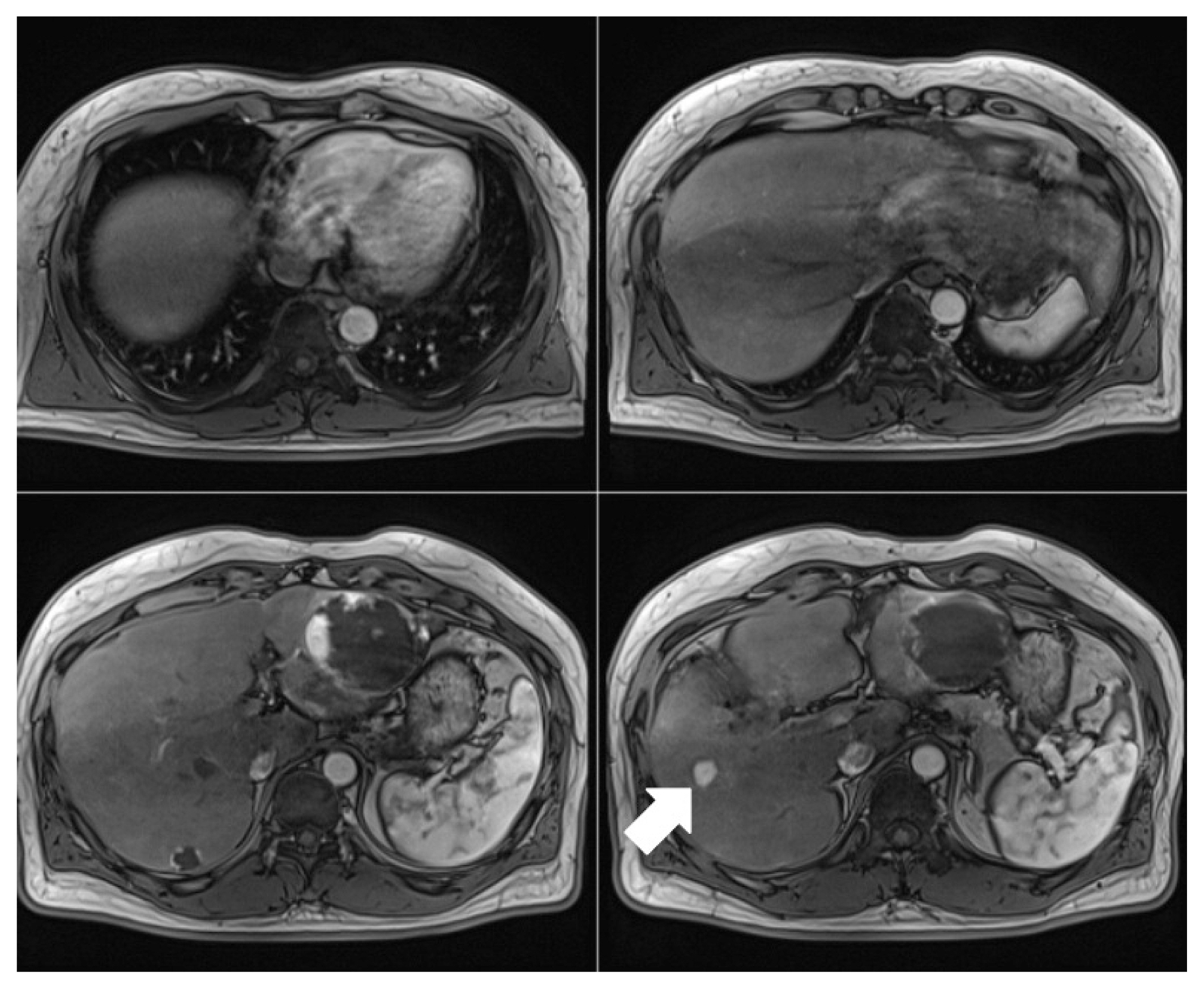
We then decided to perform radiofrequency ablation (RFA) of the S5 lesion, followed by HAIC with epirubicin (50 mg/m2, one day) and cisplatin (10 mg, seven days) in addition to the FUDR chemotherapy. After successful RFA, 3 additional cycles of HAIC were administered combined with three cycles of chemotherapy. After a single RFA and 6 cycles of HAIC, no recurrence was found in the follow-up image, and PIVKA-II level was normalized to 20.82 mAU/mL. The patient follow-up until May 2021 in the outpatient clinic showed no recurrence of HCC (Fig. 4).
DISCUSSION
According to the data obtained from the Korean Nationwide Cancer Registry, upfront transarterial therapy was administered to the largest proportion of patients (37.5%) diagnosed with HCC. Among transarterial therapies administered to the patients, conventional TACE accounted for 96.6%, making it the most frequently performed procedure.6 In case of recurrence after surgery or local ablation therapy, TACE is generally selected as the treatment, considering the patient’s residual liver function, location, and the number of recurrent masses observed. However, TACE is a palliative treatment administered repeatedly, which may decrease its therapeutic efficacy and result in deterioration of liver function. Thus, although not yet fully defined, the concept of TACE refractoriness is proposed when the disease progresses despite repeated TACE. In Japan, TACE refractoriness was defined as follows: 1) Two or more consecutive insufficient responses of the treated tumor (viable lesion > 50%) even after changing the chemotherapeutic agents and/or reanalysis of the feeding artery during response evaluation via computed tomography or MRI at 1–3 months after adequate number of selective TACE procedures; 2) the appearance of a higher number of liver lesions than in the previous TACE procedure; 3) continuous elevation of tumor markers; 4) vascular invasion; and 5) extrahepatic spread.4 A multi-tyrosine kinase inhibitor (TKI) (i.e., sorafenib or lenvatinib) is mainly to treat patients with suspected TACE refractoriness, but the efficacy is insufficient, so HAIC may be an alternative choice. In particular, through several studies in Japan, HAIC is recommended first if there is no extrapheatic spread and there is major portal invasion in the state of TACE refractoriness.4,5
HAIC delivers chemotherapeutic agents directly to the hepatic artery that feed HCC, enabling the infusion of high concentrations of chemotherapeutic agents into the tumor, thereby increasing antitumor effect. The first-pass effect in the liver can minimize systemic toxicity by generating relatively low systemic levels of chemotherapeutic agents.7 No consensus on standard treatment via HAIC is available in the absence of robust evidence of survival benefit based on randomized controlled trials. However, many clinical studies reporting the treatment of advanced HCC via HAIC demonstrated high response rates, favorable long-term outcomes, and fewer side effects.8 Thus, HAIC shows sufficient value as one of the treatment strategies for HCC.
Various combinations of cisplatin, 5-fluorouracil (5-FU), interferon, and epirubicin have been reported as chemotherapeutic regimens for HAIC, and the combination of cisplatin and 5-FU is the most commonly used.8 Among the various combinations, the combination of cisplatin, FUDR, and epirubicin was determined as the therapy for use in HAIC. FUDR instead of 5-FU was selected as the chemotherapeutic agent in HAIC because intra-arterial FUDR, which is a metabolite of fluorouracil, showed increased hepatic extraction (> 95%) and more than 10-fold intrahepatic concentration.9 Epirubicin is mainly used for systemic chemotherapy of cancers such as breast cancer, esophageal cancer, and gastric cancer, and also in TACE for HCC. Song et al.10 reported that the addition of epirubicin to the HAIC regimen resulted in more effective control of the intrahepatic tumor. The optimal regimen for the treatment of advanced HCC is still debated, underscoring the need for additional prospective randomized clinical trials to identify an effective regimen.
Because HAIC requires repeated infusion of chemotherapeutic agents at short intervals (mainly 3–4 weeks), a permanent access route to the hepatic artery is needed. In the 1980s, port implantation for HAIC was performed via laparotomy. However, advances in minimally invasive techniques led to percutaneous implantation of catheter-port systems, reducing complications and facilitating re-access in case of port dysfunction. Jens Ricke et al.11 reported that interventional hepatic arterial port placement was successful in 104 of 105 patients, and anatomic variants of the hepatic arterial blood supply were encountered in 33 of 104 patients (31.7%). Anatomic variants were overcome by ignoring the accessory hepatic artery or treating it with embolization and implanting a catheter into the splenic artery. However, 7 patients with variation in each hepatic artery that resulted in left and right hepatic artery originating from different arterial vessels could not implant the port in the above way, so they implanted two ports. Based on the foregoing study and our case, patients with vacular variation contemplating HAIC can be treated via port implantation through appropriate access.
RFA is currently the most frequently used locoregional therapy for HCC, and there was no significant difference in survival rate in patients with HCC with a single mass less than 3 cm compared to the hepatic resection group.12 In our case, RFA was performed on one lesion that increased in size after the first 3 cycles of HAIC. There were no complications after RFA, and the patient recovered quickly and was able to complete an additional 3 cycles of HAIC.
In conclusion, we describe a case involving a patient diagnosed with HCC refractory to TACE and hepatic artery variation, manifesting sustained and complete response with HAIC and RFA after port implantation in both liver lobes. Despite the lack of treatment consensus using standard HAIC, many studies have reported treatment effectiveness in some patients. A further study is needed to determine patient groups with good responses. Further, if a combination of HAIC and systemic therapies such as TKI or immuno-oncologic agents yields better results than systemic treatment alone, HAIC represents a standard treatment options.