Mucinous precursor lesions of mucinous carcinoma in breast: Incidence and histopathologic features
Article information
Abstract
Abstract
Objectives
Columnar cell lesion (CCL), atypical ductal hyperplasia (ADH) and ductal carcinoma in situ (DCIS) may be premalignant lesion of mammary invasive carcinoma. A few recent investigators reported that the precursor lesions exhibited mucin production and they might be potential precursor lesion for mucinous carcinoma (mCA). This study aims to investigate the incidence and histopathologic characteristics of mucinous precursor lesions, including mucinous DCIS (mDCIS) and mucinous CCL (mCCL).
Methods
We retrospectively analyzed invasive carcinomas with mucin. Cases were grouped into three subgroups: pure mCA, mixed mCA, and invasive carcinoma of no special type with mucin production (IC of NST-m). Precursor lesions were evaluated with PAS and alcian blue staining.
Results
Total 27 cases of invasive carcinoma with mucin were analysed and classified as 18 pure mCA, 7 mixed mCA, and 2 IC of NST-m. mDCISs were found in 12 pure mCA, 4 mixed mCA and 2 IC of NST-m. mCCLs were found in 7 pure mCA and 2 mixed mCA. Majority of mucin was identified in both cytoplasm and ductal lumen, while some tumors exhibited only cytoplasmic mucin. We also observed three patterns of mDCIS classifiable by location of mucin and architecture of tumor cells.
Conclusions
Cytoplasmic mucin suggested that mucinous feature of precursor lesions in the vicinity of mCA might not be a passive morphologic finding but be involved in development of mCA.
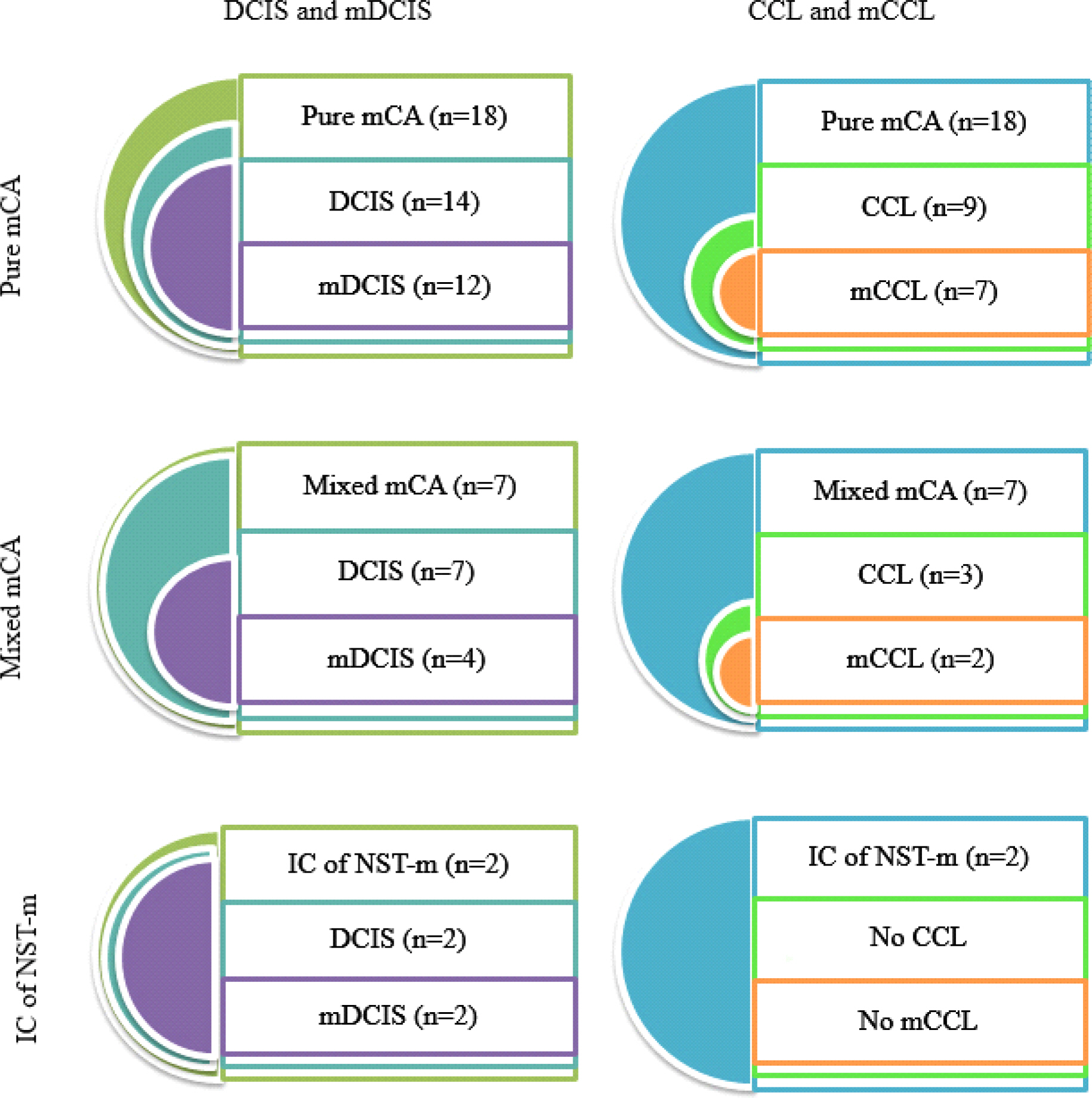
Distribution of ductal carcinoma in situ, mucinous ductal carcinoma in situ, columnar cell lesion and mucinous columnar cell lesion is variable in each groups of invasive carcinoma with mucin. (DCIS, ductal carcinoma in situ; mDCIS, mucinous ductal carcinoma in situ; CCL, columnar cell lesion; mCCL, mucinous columnar cell lesion; mCA, mucinous carcinoma; IC of NST-m, invasive carcinoma of no special type with mucin production)

Mucinous ductal carcinoma in situ is histologically classified into three patterns. (A) In pattern 1, ductal carcinoma in situ exhibits ducts overextended by intraluminal mucin. (B) They are partially lined by attenuated ductal epithelium. (C, D) In pattern 2, ductal carcinoma in situ exhibits intraluminal mucin without ductal overextension. (E, F) In pattern 3, ductal carcinoma in situ exhibits minute intercellular spaces or intracytoplasmic vacuoles filled with mucin without distinct ductal mucin. (Hematoxylin-Eosin stain, A, C, E: x40, B, D, F: x400)
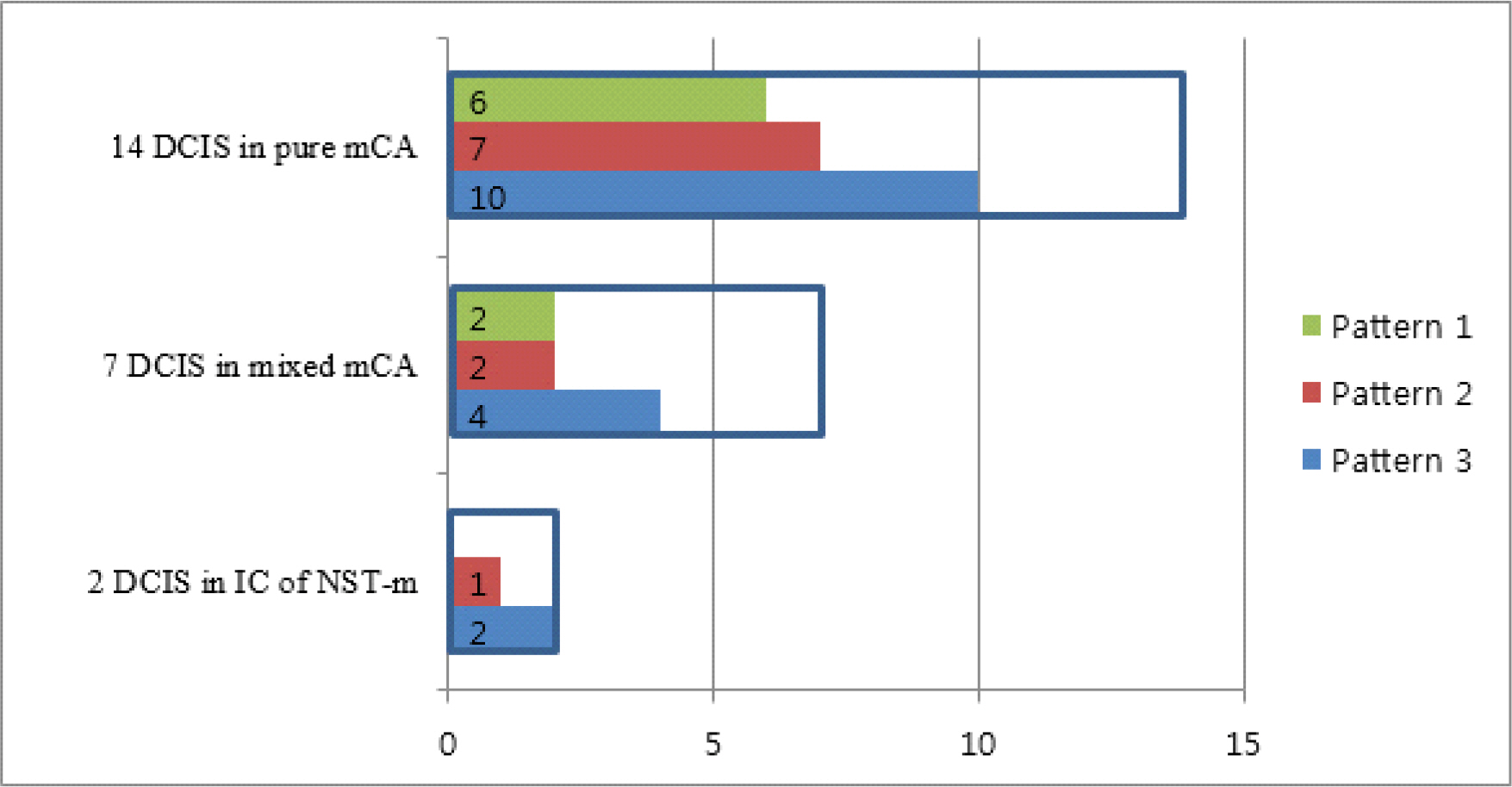
Three patterns of mucinous ductal carcinoma in situ are identified in three groups of invasive carcinoma with mucin. Some cases exhibit more than one pattern of mucinous ductal carcinoma in situ, and most common pattern is pattern 3. (DCIS, ductal carcinoma in situ, mCA, mucinous carcinoma; IC of NST-m, invasive carcinoma of no special type with mucin production)

