References
1. Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology 1975;68:105–12.
2. Jang JW. Management of Patients with Hepatitis B Virus Infection Who Receive Immunosuppressive Treatment or Chemotherapy. Korean J Med 2012;82:149–58.
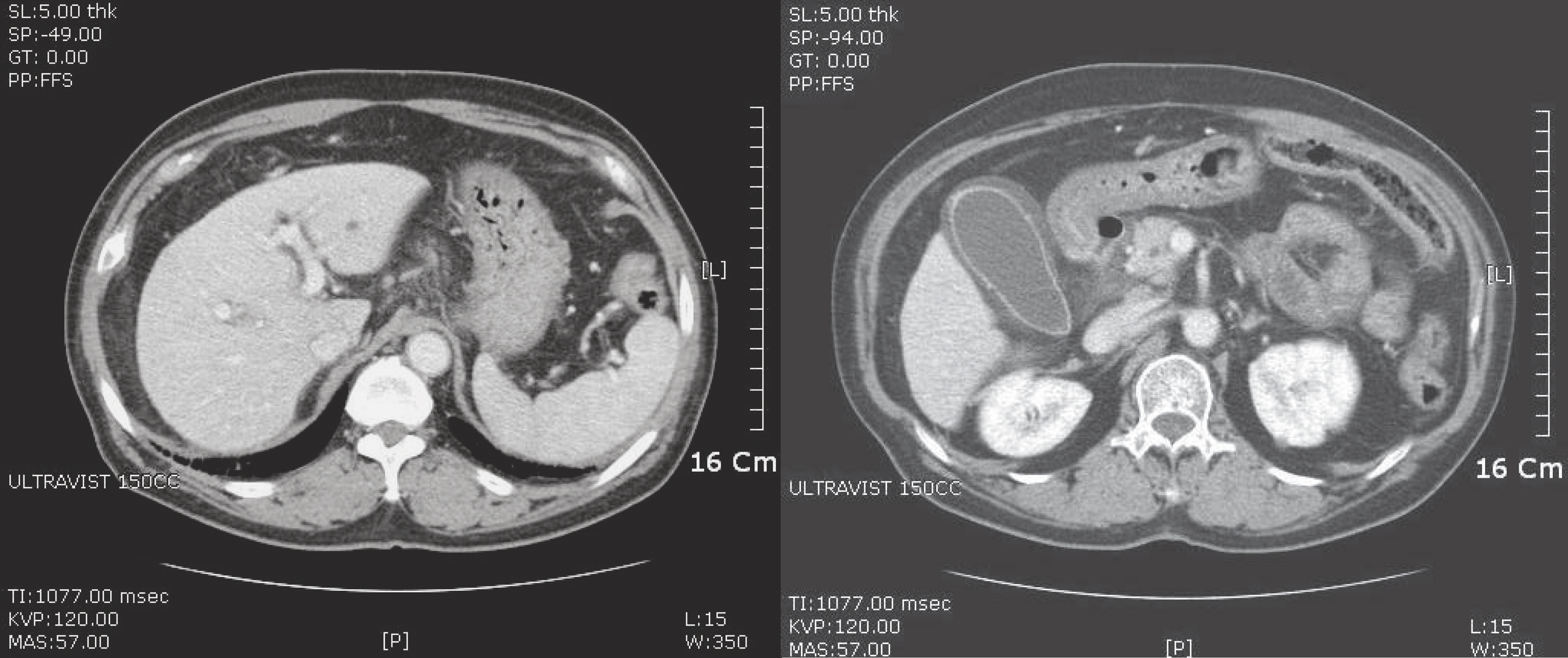
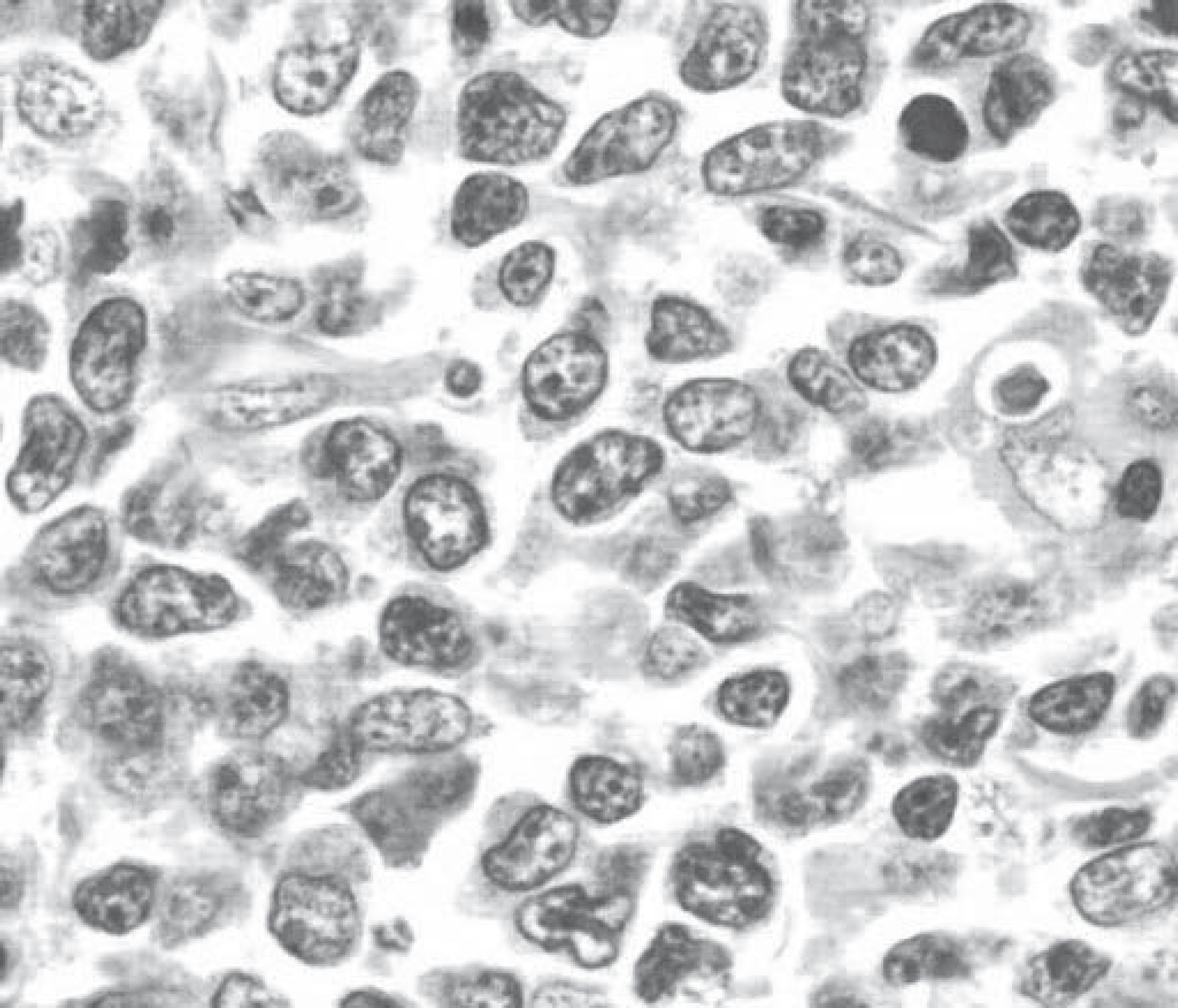
3. Lim SM, Jang JW, Kim BW, Choi H, Choi KY, Park SJ, et al. Hepatitis B vims reactivation during chlorambucil and prednisolone treatment in an HBsAg-negative and anti-HBs-positive patient with B-cell chronic lymphocytic leukemia. Korean J Hepatol 2008;14:213–8.
4. Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients'recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med 1996;2:1104–8.
5. Song SH, Hwang SG. Occult hepatitis B virus infection: transmission and reactivation. Korean J Gastroenterol 2013;62:148–53.
6. Kusumoto S, Tanaka Y, Mizokami M, Ueda R. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol 2009;90:13–23.
7. Lee KS, Kim DJ. Korean Association for the Study of the Liver Guideline Committee. Management of Chronic Hepatitis B. Korean J Hepatol 2007;13:447–88.
8. Chung SM, Sohn JH, Kim TY, Yoo KD, Ahn YW, Bae JH, et al. Fulminant hepatic failure with hepatitis B virus reactivation after rituximab treatment in a patient with resolved hepatitis B. Korean J Gastroenterol 2010;55:266–9.
9. Gupta S, Govindarajan S, Fong TL, Redeker AG. Spontaneous reactivation in chronic hepatitis B: patterns and natural history. J Clin Gastroenterol 1990;12:562–8.
10. Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology 2006;43:209–20.
11. Park JW, Park KW, Cho SH, Park HS, Lee WJ, Lee DH, et al. Risk of hepatitis B exacerbation is low after transcatheter arterial chemoembolization therapy for patients with HBV-related hepatocellular carcinoma: report of a prospective study. Am J Gastroenterol 2005;100:2194–200.
12. Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology 2006;43:233–40.
13. Jung HM, Jun DW, Min JY, Doo EY, Nam KW, Kwon YI, et al. A Case of Acute Hepatitis B by Occult HBV Infection without HbsAg Seroconversion. Korean J Med 2012;83:619–23.
14. Lau GK. Hepatitis B reactivation after chemotherapy: two decades of clinical research. Hepatol In 2008;2:152–62.
15. Kusumoto S, Tanaka Y, Ueda R, Mizokami M. Reactivation of hepatitis B virus following rituximab-plus-steroid combination chemotherapy. J Gastroenterol 2011;46:9–16.
16. Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology 1991;100:182–8.
17. Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology 2003;125:1742–9.
18. Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci USA 1986;83:1627–31.
19. Kawsar HI, Shahnewaz J, Gopalakrishna KV, Spiro TP, Daw HA. Hepatitis B Reactivation in Cancer Patients; Role of Prechemotherapy Screening and Antiviral Prophylaxis. Clin Adv Hematol Oncol 2012;10:370–8.
20. Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu HL, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009;27:605–11.
21. The Korean Association for the Study of the Liver. Guidelines for Chronic hepatitis B [Internet] Seoul: The Korean Association for the Study of the Liver; c2011. [cited 2011 DecOl]. Available from: http://www.kasl.org/html/sub05_03_l.asp.
22. Zelenetz Andew D., Gordon Leo I., Wierda William G., Abramson Jeremy S., Advani Ranjana H., Babis Andreadis C., et al. NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin's lymphomas. J Natl Compr Cane Netw. Version 1 2014.
23. Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med 2001;344:68–9.
24. Sera T, Hiasa Y, Michitaka K, Konshi I, Masuura K, Tokumoto Y, et al. Anti-HBs-positive liver failure due to hepatitis B virus reactivation induced by rituximab. Intern Med 2006;45:721–4.
25. Kim EB, Kim DS, Park SJ, Park Y, Rho KH, Kim SJ. Hepatitis B virus reactivation in a surface antigen-negative and antibody-positive patient after rituximab plus CHOP chemotherapy. Cancer Res Treat 2008;40:36–8.