Basic knowledge of endoscopic retrograde cholangiopancreatography
Article information
Abstract
Endoscopic retrograde cholangiopancreatography (ERCP) was first performed in the late 1960s. Due to advancements in instruments, devices, and techniques, ERCP has played an important role in the management and diagnosis of pancreatobiliary disorders. However, ERCP is accompanied by the risk of various complications even if performed by an expert. The incidence of ERCP complications is approximately 4% to 10%, while the incidence of fatal complications, such as death, is less than 0.5%. To prevent adverse events, experts performing ERCP must recognize and address ERCP-related complications and understand the various techniques. In this review, we summarize the complications and techniques of ERCP.
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP), first attempted in late 1960's, has become the most important procedure for the treatment and diagnosis of various pancreatic disorders and biliary diseases. Moreover, ERCP is actively being implemented in many hospitals. Despite the importance of ERCP, the procedure has the limitation of being high risk compared to endoscopic other procedures and it may be accompanied by various complications. Even ERCP performed by experts cannot completely avoid the occurrence of complications. Thus, to minimize potential complications and ensure the safe execution of the procedure, it is crucial to master various techniques and become well-acquainted with how to manage the diverse complications that may arise effectively. The incidence of ERCP complications is reported to be about 4%–10%, and among these, life-threatening fatal complications are known to be less than 0.5%. In this review, we briefly discuss the comprehensive contents, complications, and coping methods of various guidelines for ERCP, which is an essential procedure in the biliary and pancreatic fields [1-3].
ERCP indication
ERCP can cause several procedure-related complications with severe complications occurring in 0.5% of cases. Hence, selecting patients judiciously is vital, ensuring that the procedure is conducted solely for those who unequivocally require it. In the past, ERCP was used for both treatment and diagnosis; however, with the development of noninvasive endoscopic ultrasound (EUS) or magnetic resonance cholangiopancreatography, ERCP for diagnostic purposes has not been performed except in special cases and is mainly performed for therapeutic purposes only. The indications and contraindications for ERCP, as recognized by the American Society for Gastrointestinal Endoscopy, are listed in Table 1 [4].
Selective biliary cannulation
For the successful performance of ERCP, whether for therapeutic or diagnostic purposes, the first essential step is effective selective biliary cannulation. Selective biliary cannulation does not always guarantee success and is the most common cause of ERCP failure. As the number of biliary cannulation attempts increases, the incidence of ERCP complications also increases.
In a randomized controlled study evaluating the efficacy of selective biliary cannulation using the sphincterotome and ERCP catheter, the success rate was reported as 84% for the sphincterotome, while the ERCP catheter demonstrated a success rate of 62%, indicating a notable difference in performance between the two methods (p<0.05) [5]. The reason for this result is that the sphincterotome has a relatively flexible tip, and the angle of the tip can be adjusted to enable a look-up position; therefore, adjusting the tip to the appropriate axis is advantageous. In addition, the sphincterotome did not demonstrate an increase in complications compared with the ERCP catheter (p=0.30); therefore, selecting the sphincterotome for successful selective biliary cannulation displayed more advantageous results than the ERCP catheter [6].
Endoscopic sphincterotomy
Endoscopic sphincterotomy (EST) is a procedure involving cannulation of the sphincterotome into the bile duct via the major papilla and incision using a high-frequency current. According to a meta-analysis comparing ERCP using EST and surgical removal of biliary stones, no statistically significant difference was observed in the biliary stone removal, complication, and mortality rate [7].
According to the European Society of Gastrointestinal Endoscopy ERCP complication guidelines presented in 2020, EST is a high-bleeding-risk procedure and is relatively contraindicated in patients consuming antiplatelet agents, such as clopidogrel, receiving new oral anticoagulants, and patients with acute pancreatitis [8]. According to a large prospective study on nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin, the two drugs were not reported to be bleeding risk factors after EST [9]. Additionally, continued administration or discontinuation of antiplatelet medication did not exhibit a statistically significant effect on the occurrence of bleeding after EST (continuation odds ratio [OR], 0.67; 95% confidence interval [CI], 0.21–2.11; withdrawal OR, 1.25; 95% CI, 0.90–1.74) [10]. However, antiplatelet drugs from the non-acetylsalicylic acid group should be discontinued [8].
The risk factors for EST site bleeding include antiplatelet medication use, increased international normalized ratio, decreased platelet count, liver cirrhosis, heart disease, hypertension, and chronic kidney disease [11]. Post-EST bleeding can be prevented by correcting possible risk factors before performing EST [11]. Bleeding risk also varies depending on the type of radiofrequency used during EST. Using a coagulation wave simultaneously, or the Endocut mode is safer than using a cutting wave alone. The simultaneous use of the Endocut mode or coagulation wave has demonstrated a reduction in the risk of zipper cuts, bleeding, and post-ERCP pancreatitis [12]. On the other hand, no difference was observed in bleeding risk depending on the type of sphincterotome used. Moreover, in a comparative study between the blade lengths of 20 mm and 30 mm of the sphincterotome, no statistically significant difference was identified between the two groups [12].
The distribution of blood vessels, especially arteries, in the ampulla of Vater is low at 10% to 11% in the 10–11 o'clock direction. Consequently, research has indicated that maintaining the incision direction of the EST in the 11–12 o'clock direction is preferable to minimize the risk of bleeding [13]. The incision sizes were divided into small, medium, and large. An incision below the transverse fold was classified as a small incision; an incision up to the superior margin of the papillary bulge was a large incision; and an incision in the middle, as a medium incision (Fig. 1).
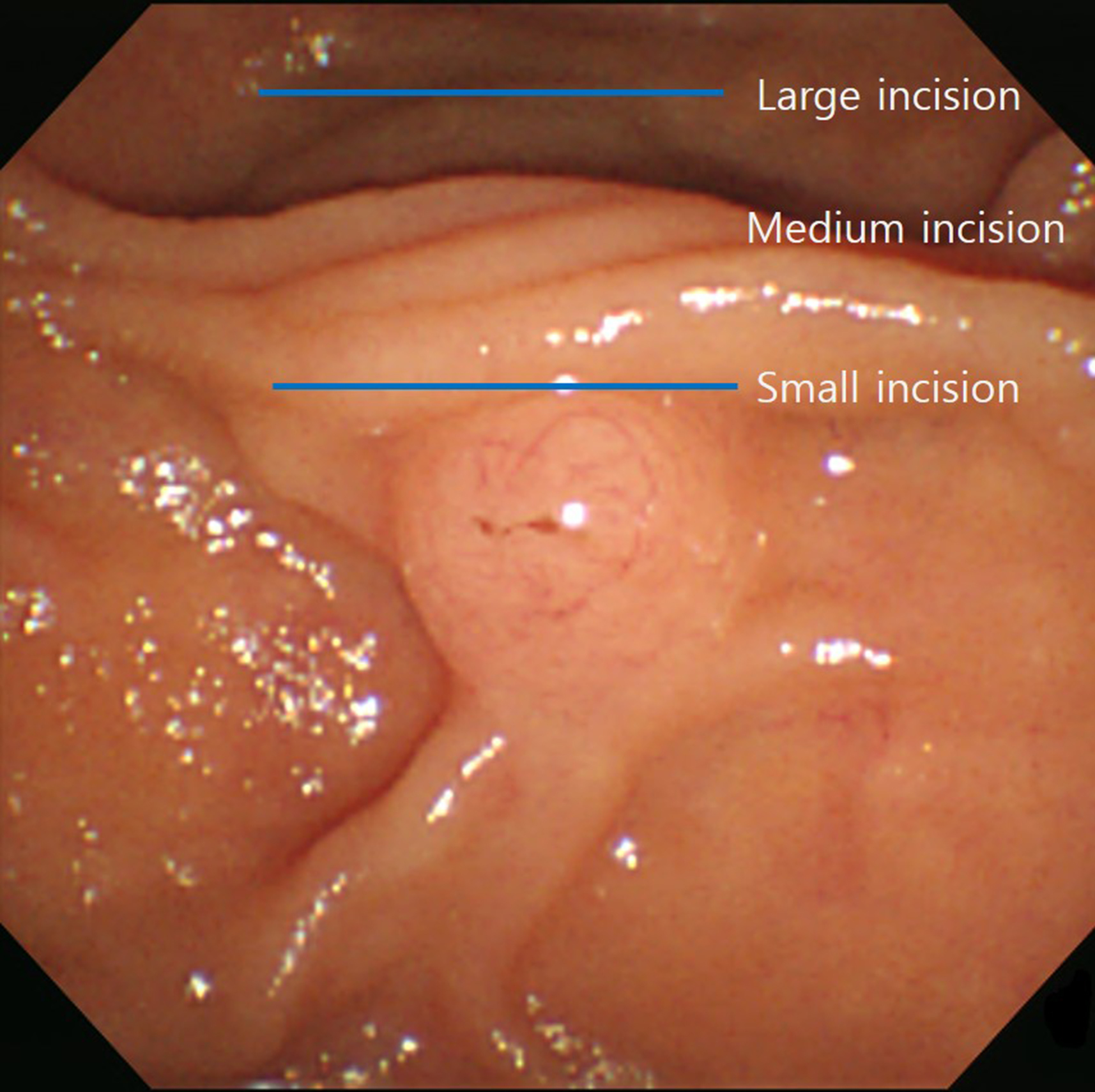
An incision below the transverse fold was classified as a small incision; an incision up to the superior margin of the papillary bulge was a large incision; and an incision in the middle, as a medium incision.
The superior sphincter of the ampulla of Vater extends into the bile duct in the lateral wall of the duodenum. The middle sphincter is located at the same level as the superior margin of the papillary bulge and protrudes into the duodenal lumen of the ampulla. Therefore, when an incision is made beyond the superior sphincter of the ampulla of Vater, the risk of duodenal perforation increases. To prevent this, incising the ampulla of Vater below the superior sphincter is important.
The high-frequency currents used during EST may interfere with or fail by implantable cardioverter-defibrillators or cardiac pacemakers; therefore, patients with implanted devices should consult a cardiologist before EST [14]. For patients with complete heart block, changing the mode to an asynchronous setting before EST is safe [14]. Additionally, electrocardiography, oxygen saturation monitoring and blood pressure must be performed before, during, and after the ERCP procedure [14].
According to a study on the timing of ERCP in biliary pancreatitis, no statistical difference in the incidence of systemic and local complications was observed (relative risk [RR], 0.59; 95% CI, 0.31–1.11 and RR, 0.86; 95% CI, 0.52–1.43, respectively) in the comparison between the group that underwent ERCP within 72 hours and the group the underwent ERCP after 72 hours following conservative treatment. Similarly, no significant differences were identified in mortality (RR, 0.74; 95% CI, 0.18–3.03) [15]. However, in the case of biliary pancreatitis accompanied by cholangitis, it was reported that systemic and local complications (RR, 0.37; 95% CI, 0.18–0.78 and RR, 0.45; 95% CI, 0.20–0.99) and mortality rates (RR, 0.20; 95% CI, 0.06–0.68) were statistically significantly low in the early ERCP group. Additionally, in patients with cholangitis accompanied by bile duct obstruction, the early ERCP group had a significantly low incidence of local complications (RR, 0.54; 95% CI, 0.32–0.91) [15]. For this reason, performing ERCP early when biliary pancreatitis is accompanied by cholangitis is strongly recommended.
Rescue technique
In cases where selective biliary cannulation is difficult, precutting sphincterotomy is effective in increasing the rate of deep selective biliary cannulation. However, precutting sphincterotomy increases the incidence of perforation and bleeding along with the incidence of post-ERCP pancreatitis [16]. The precutting sphincterotomy was incised from the upper part of the ampulla of Vater, in the proximal or distal direction. The precutting sphincterotomy is attempted in 45%–38% of all ERCPs, and the success rate of selective biliary cannulation is reported to be 35%–96% [17]. Performing precutting sphincterotomy after the insertion of an endoscopic retrograde pancreatic drainage (ERPD) stent allows for an incision of the EST while verifying the direction of the main pancreatic duct, effectively preventing the development of post-ERCP pancreatitis [18]. As precutting sphincterotomy has a higher complication incidence compared to EST, the procedure is recommended when selective biliary cannulation with EST is not possible.
When a guidewire is cannulated in the pancreatic duct during a selective cannulation attempt, selective biliary cannulation using another guidewire without removing the first guidewire is called the double-guidewire technique (Fig. 2). The following three effects can be achieved using the double-guidewire technique: first, straightening of the common channel, second, stabilization of the papilla, and third, moving the guidewire in the distal direction has the effect of opening the bile duct orifice, making biliary cannulation easier [17].

When a guidewire is cannulated in the main pancreatic duct during a selective cannulation attempt, selective biliary cannulation using another guidewire without removing the first guidewire is called the double-guidewire technique.
In cases where selective biliary cannulation fails, another rescue technique is the rendezvous technique using EUS, which has recently been actively attempted. After puncturing the extrahepatic bile duct using a 19-gauge needle under EUS guidance, a short guidewire, 260 cm in length, was inserted and passed through the papilla. Next, the scope was changed from EUS to duodenoscopy, and selective biliary cannulation was attempted [19]. According to a retrospective comparative study, the EUS-guided rendezvous technique reported a better biliary cannulation success rate than precutting sphincterotomy (98.3% vs. 90.3%, p=0.03). Additionally, no significant difference was observed in complication rates between the two techniques (3.4% vs. 6.9%, p=0.27) [19]. The transgastric approach and short scope position, which involve the observation and puncturing of the bile duct through EUS in the stomach, offer the benefit of enabling the procedure to be performed with a straightened endoscope. Other methods include the transduodenal approach, long-scope position, and the push method. In this method, the procedure is performed by puncturing the bile duct in the duodenal bulb using EUS and then pushing the endoscope along the gastric greater curvature (Fig. 3) [20]. The selection of the puncture location was based on the anatomical condition.
Altered anatomy ERCP
Even in patients with altered gastric and duodenal anatomy due to gastric cancer operation, the EST indication is the same as that in patients with normal anatomy. In patients who have undergone Billroth I type subtotal gastrectomy, EST is performed in the same manner as in patients with normal anatomy. In patients who have undergone Billroth II type subtotal gastrectomy, either a side-viewing endoscope or a forward-viewing endoscope can be used. The choice is determined by the endoscopist's skill level and the patient's anatomy [21]. In cases where access to the papilla is difficult with both a side- and forward-viewing endoscope, the next procedure may be performed after accessing the papilla using a balloon enteroscope [22]. In cases of Billroth II subtotal gastrectomy, a special type of EST knife, such as a push-type sphincterotome, is used as the papilla is accessed from the anal side [22].
No significant difference was observed in a randomized controlled trial on the success rate of EST using a needle knife between the side-viewing and forward-viewing endoscopes in patients with altered anatomy (83% vs. 80%). Additionally, no significant difference was observed in EST-related complications [23]. However, complications related to endoscope insertion occurred more commonly with side-viewing endoscopes compared to forward-viewing ones (0% vs. 18%, p<0.05) [23].
In patients who have undergone Roux-en-Y surgery, accessing the papilla using an endoscope of normal length is difficult [24]. In this case, a balloon enteroscopy can successfully access the papilla in 85%–95% of cases [22]. In patients with altered anatomy, the procedure is sometimes performed by expanding the bile duct to 6–8 mm using only endoscopic papillary balloon dilation without EST. In a randomized controlled trial comparing EST and endoscopic papillary balloon dilation alone, no difference in procedure time, frequency of mechanical lithotripsy, or complication rate between the endoscopic papillary balloon dilation and EST groups was identified [25].
Periampullary diverticulum
When the duodenum is accompanied by a periampullary diverticulum, the length and direction of EST must be carefully selected because selective biliary cannulation is difficult and complications easily occur depending on the shape of the papilla and periampullary diverticulum. Bleeding complications occurred more often after EST in patients with periampullary diverticula than in those without periampullary diverticula (8.8% vs. 4.8%, p=0.039) [26]. In particular, when the papilla is located inside a periampullary diverticulum, such as in periampullary diverticulum type 1 (Fig. 4), using the two-devices-in-one-channel method is helpful. The two-devices-in-one-channel method involves inserting forceps for biopsy through the working channel of the endoscope, grabbing the duodenal mucosa, moving it to the distal portion, and moving the papilla outside the periampullary diverticulum to fix it. Subsequently, selective biliary cannulation was attempted by inserting the ERCP catheter through the working channel [27]. Another method involves the use of an endoscopic clip to move the papilla outside the periampullary diverticulum, fix it, and proceed with the procedure [28].
ERCP-related complications
Table 2 displays various complications related to ERCP summarized in the European Society of Gastrointestinal Endoscopy guideline published in 2020 [8]. Early complications related to EST include cholangitis, pancreatitis, perforation, and bleeding. Additionally, complications are reported to occur in 3.0%–11.8% of cases. The incidence of complications is reported to be 0.5%–6.9% for acute pancreatitis, 0%–27.0% for bleeding, 0%–1.8% for perforation, and 0%–4.2% for cholangitis [8].
1. Post-ERCP pancreatitis
The risk factors related to the occurrence of post-ERCP pancreatitis include pancreatic congestion, increased pancreatic duct pressure due to papillary edema, injection of contrast medium into the main pancreatic duct, damage to the main pancreatic duct due to devices, and thermal damage to the pancreatic duct orifice. The occurrence of post-ERCP pancreatitis is believed to be the result of a complex effect of several factors rather than a single factor [29]. Patient-related risk factors include female sex, young age (<35 years), history of pancreatitis and Oddi’s sphincter dysfunction, which are known to be independent risk factors [30]. According to population-based studies, EST has also been reported as an independent risk factor for post-ERCP pancreatitis [31].
Various methods for reducing the occurrence of post-ERCP pancreatitis include prophylactic ERPD stent insertion and rectal NSAID suppositories. Administration of pancreatic enzyme inhibitors, somatostatin, octreotide, nitroglycerin, and epinephrine spray to the papilla was ineffective in preventing post-ERCP pancreatitis [32]. The 2020 European Society of Gastrointestinal Endoscopy guidelines recommend that 100 mg of diclofenac or indomethacin should be administered via the transrectal route immediately before the procedure in all patients undergoing ERCP without contraindications [8].
By comparing various NSAID administration routes, only the transrectal route was discovered to be effective [8]. In a study on the timing of NSAID administration, pancreatitis occurred in 6% of patients where NSAIDs were administered before ERCP versus 12% of patients where NSAIDs were administered after ERCP, demonstrating that NSAID administration before ERCP as opposed to after ERCP was more effective in preventing post-ERCP pancreatitis (RR, 0.47; 95% CI, 0.27–0.82) [33]. In patients receiving a single 100 mg dose of indomethacin or clopidogrel, aspirin did not increase the bleeding risk after EST [34].
Additionally, in cases with a high risk of post-ERCP pancreatitis, such as main pancreatic duct cannulation of the guidewire, the double-guidewire technique during ERCP or administration of contrast medium into the main pancreatic duct, insertion of a prophylactic ERPD stent is recommended [8]. In eight meta-analyses published between 2011 and 2019, prophylactic ERPD stent insertion was associated with a reduced incidence of post-ERCP pancreatitis (OR, 0.22–0.39) [8]. Furthermore, in a meta-analysis related to severe post-ERCP pancreatitis, the incidence of post-ERCP pancreatitis was significantly reduced (OR, 0.22–0.26) [8]. Cost-effectiveness analysis displayed that using prophylactic ERPD stent insertion only in high-risk patients was the most cost-effective strategy [8]. For preventive ERPD stent insertion, the use of a flange-type or 5-Fr pigtail-type stent is recommended, and the ERPD stent should be removed within 5 to 10 days [8].
The European Society of Gastrointestinal Endoscopy guidelines also recommend active fluid therapy with lactated Ringer's solution (3 mL/kg/hr during ERCP, 20 mL/kg bolus after ERCP, and 3 mL/kg/hr for 8 hours after ERCP) in patients contraindicated for NSAIDs who are not at risk of volume overload, such as heart failure or renal failure, and who cannot receive an ERPD stent [8].
2. Bleeding
In the 2020 European Society of Gastrointestinal Endoscopy guidelines, the risk factors for bleeding were defined as platelet count <50,000/mm3, dialysis for chronic kidney disease, anticoagulant use, liver cirrhosis, and bleeding during ERCP [8]. When bleeding occurs, endoscopic hemostasis is considered the first treatment. Severe bleeding requiring transfusion of more than five units of red blood cells or endoscopic hemostasis occurs in 0.1%–0.5% of the cases [35]. Endoscopic hemostasis includes local compression, injection, coagulation, and clipping [36]. Local compression using a balloon catheter or endoscopic papillary balloon dilatation is relatively effective and simple technique. The local hypertonic saline-epinephrine solution is also relatively effective and safe but can cause pancreatitis due to mucosal damage and edema, and perforation can also occur; therefore, caution is required during the procedure [37]. Other methods include heat probing, argon plasma coagulation, and hemostatic clipping, but they must be performed with caution to prevent damage to the main pancreatic duct orifice [38].
3. Perforation
Risk factors for perforation include the precutting method, presence of papillary lesions, dilated bile duct, sphincter of Oddi dysfunction, EST, dilatation of bile duct stricture, and altered anatomy [8]. In particular, retrospective studies have displayed that the anatomy of Billroth II type subtotal gastrectomy is associated with an increased incidence of bowel perforation [39]. If perforation is suspected, abdominal computed tomography should be performed. Perforation requiring surgical treatment occurs in 0.2%–0.7% of cases and death has been reported in 0.2%–0.3% of cases [9]. If the size of the perforation is small, it often improves through conservative treatments such as nasogastric tube drainage, pancreatic duct drainage, and biliary drainage [40]. However, if no improvement is observed within 24 hours of conservative treatment, surgical treatment is required. A delay in the treatment and diagnosis can result in a poor prognosis [40].
Plastic stent or self-expandable metal stent insertion
The 2020 European Society of Gastrointestinal Endoscopy guidelines recommend not performing routine EST when inserting a single plastic or self-expandable metal stent [8]. Routinely use of prophylactic antibiotics before ERCP was also not recommended. However, antibiotic administration is recommended in cases where incomplete biliary drainage is expected, severe immunosuppression is observed, or cholangioscopy is performed [8]. In addition, it is not always necessary to perform a blood coagulation test before ERCP who do not use anticoagulants and do not have jaundice [8]. In cases of post-ERCP pancreatitis, ERPD stent insertion should be considered only if the patient presents with severe abdominal pain, amylase elevation exceeding 10 times the normal value, or elevated levels of C-reactive protein or white blood cells [8]. If bleeding continues despite general hemostasis after EST, temporary insertion of a fully covered self-expandable metallic stent is recommended [8].
Biliary stone removal rate
A randomized comparative study of the biliary stone removal rates between EST and endoscopic papillary balloon dilation demonstrated that the biliary stone removal rate was higher in the EST group compared to the endoscopic papillary balloon dilation group. Additionally, the biliary stone removal rate during the first session was reported to be 56.2%–92.7%, and the complete biliary stone removal rate was reported to be 86.8%–100% [41]. In the case of a study comparing EST and endoscopic papillary large balloon dilatation, no significant difference was observed [42]. The biliary stone recurrence rate after EST was reported to be 4.1%–17%, the 5-year recurrence rate was 9.6%, and the 10-year recurrence rate was 13.2%. The risk factors for biliary stone recurrence include presence of gallbladder stones, edema within the bile duct, mechanical lithotripsy performed during ERCP, periampullary diverticulum, and dilatation of the bile duct [43]. The recurrence rate of biliary stones increased with the number of recurrences, rapidly rising to 23.4% after the first recurrence and 33.4% after the second recurrence [44]. In case of biliary stone recurrence, treatment through endoscopy is recommended initially. In addition, gallbladder stones can be partially prevented by cholecystectomy [45]. Cholecystectomy is particularly effective in young patients, and the RR is known to be 1.26 for those over 70 years of age, but 3.20 for those under 50 years of age [44]. Incomplete biliary stone removal, age >60 years, previous ERCP, hepatic hilar portion obstruction, and primary sclerosing cholangitis were independent risk factors for the development of cholangitis after ERCP. Conversely, complete removal of extrahepatic bile duct stones reduces the incidence of cholangitis [46].
Conclusions
ERCP is an important procedure in the treatment and diagnosis of various pancreatic and biliary diseases. However, the procedure is difficult, and in rare cases, can cause serious complications. Furthermore, the aforementioned procedure can put the patient at risk if appropriate measures are not undertaken. Therefore, an accurate understanding of biliary and pancreatic diseases should be a priority, and patients suitable for the procedure should be selected. The endoscopist must have appropriate ERCP performance skills, understand various techniques, and perform safe procedures according to the guidelines. Additionally, if a complication occurs, the endoscopist must respond appropriately. In cases where endoscopy is difficult to perform, close multidisciplinary cooperation, such as vascular embolization or surgery, is necessary. In this paper, we have summarized the contents of various ERCP-related guidelines and hope that these will be helpful to doctors performing ERCP in the future.
Summary
ERCP is a basic procedure for the treatment and diagnosis of various pancreatic and biliary diseases. However, ERCP has high-risk factors and may be associated with several complications. Accordingly, endoscopists must have appropriate ERCP performance skills, understand various techniques, perform safe procedures according to guidelines, and respond appropriately to complications that might occur.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
All the work was done by JWL.