Comparison of circuit patency and exchange rates between the original and generic versions of nafamostat mesylate in critically ill adults receiving continuous renal replacement therapy
Article information
Abstract
Background
Nafamostat mesylate is widely used as an anticoagulant in continuous renal replacement therapy (CRRT). The generic versions of nafamostat mesylate have identical main components to the original product. However, it is questionable whether the generic versions have the same efficacy as the original. Therefore, we compared the circuit patency and exchange rates of the original nafamostat mesylate and a generic version to determine which is more efficient as an anticoagulant in CRRT.
Methods
This retrospective study enrolled 1,255 patients hospitalized to receive CRRT who received the original version of nafamostat mesylate or a generic version between January 2010 and July 2018. We evaluated the filter lifespan, number of filters used per day, mean blood flow, and transmembrane pressure (TMP).
Results
The mean filter lifespan was 36.3±15.1 hours in the original product group and 22.2±16.2 hours in the generic product group, which was not a statistically significant difference (p=0.060). The mean TMP was 62.2±47.3 mmHg in the original product group and 74.5±45.6 mmHg in the generic product group (p=0.045).
Conclusions
This retrospective study suggests no meaningful difference in filter lifespan between the original and generic versions of nafamostat mesylate. However, TMP was lower in the original product group than in the generic product group.
Introduction
Continuous renal replacement therapy (CRRT) is an effective kidney replacement treatment in patients with hemodynamically unstable acute kidney injury (AKI) [1]. Over the years, CRRT has become easier and safer to adopt because of advances in science and technology [2]. One of the advanced techniques is to minimize coagulation of the circuit filter, often through use of anticoagulants. Among them, heparin has been widely used for CRRT [3]. Heparin in CRRT produces some adverse effects, including bleeding events and heparin-induced thrombocytopenia [4]. Therefore, several studies have explored other anticoagulants to minimize the risk of bleeding and compared them with heparin (e.g., regional citrate anticoagulation, thrombin antagonists, prostacyclin anticoagulants) [1,3,5-7].
Nafamostat mesylate (6-amino-2-naphthyl p-guanidinobenzoate dimethane sulfonate; Futhan, SK Chemicals) is a prostacyclin analog that inhibits serine proteases. The half-life of nafamostat mesylate is 8 minutes, and it is eliminated quickly from the blood. Therefore, nafamostat mesylate can be used for people at high risk of bleeding [6]. At least one generic version of nafamostat mesylate has been released and is in use. These generic versions have identical main components to those of the original product; however, due to differences in additives and impurities, it is unclear whether they have the same efficacy. In this study, we conducted a comparison of circuit patency and exchange rates between original and generic versions of nafamostat mesylate among AKI patients receiving CRRT.
Methods
Ethical statements: This study was approved by the Institutional Review Board of Kosin University Gospel Hospital (IRB No. KUGH 2018-10-021). The written informed consent requirement was waived.
1. Patients
We conducted a retrospective study to compare the original nafamostat mesylate with generic versions, focusing on CRRT procedure time. In this single-center, unblinded, non-randomized study, 1,255 patients (aged 18–80 years) hospitalized in the intensive care unit for CRRT who received the original version of nafamostat mesylate or a generic version were enrolled between January 2010 and July 2018 at one of three tertiary hospitals.
2. Criteria
Patients were included in this study if they were diagnosed with AKI, aged 18 to 80 years, and considered by the medical team to require CRRT. All patients met at least one of the following criteria: (1) increase in serum creatinine >1.5 times above baseline; (2) glomerular filtration rate decreased by >25%; or (3) urine volume decreased to <0.5 mL/kg/hr for >6 hours. Patients who met any of the following criteria were excluded: (1) CRRT performed for <24 hours; (2) weight <50 kg or >120 kg; (3) undergoing CRRT due to a non-kidney indication; (4) receiving dialysis under a diagnosis of end-stage renal disease; (5) CRRT started <24 hours after hospitalization.
We excluded patients identified within 24 hours of hospitalization because of the many changes in anticoagulant dosing during this time of CRRT.
3. Clinical data collection
Clinical data on patient demographics, CRRT operating characteristics, and CRRT daily performance were collected at the beginning of the procedure. Laboratory testing, including blood urea nitrogen and creatinine measurements, also was performed at initiation of CRRT.
4. Study outcomes
The primary outcome was filter lifespan and number of filters used per day in AKI patients on CRRT receiving original or generic versions of nafamostat mesylate. The secondary outcome was mean blood flow and transmembrane pressure (TMP).
5. CRRT modality
Patients who received CRRT in this study were treated in continuous venovenous hemodiafiltration mode. We used a Prisma dialysis machine (Gambro), and selected membrane dialyzers were ST100 (acrylonitrile + sodium methallyl sulfonate + polyethylene imine) or M100 (acrylonitrile + sodium methallyl sulfonate). A 12-French dual lumen catheter was used for central vein access and inserted into the internal jugular or femoral vein. Bicarbonate-buffered replacement solutions were used and delivered in post-dilution mode.
The starting dose of nafamostat mesylate was 20 mg/hr, which was adjusted as needed by 20–30 mg/hr to achieve an activated clotting time of 150 to 200 seconds. Filters were exchanged every 48 hours or when they failed due to clotting.
6. Statistics
All variables were analyzed using SPSS for Windows version 23.0 (IBM Corp.). Continuous variables are expressed as mean±standard deviation (SD). Categorical variables are expressed as frequency and percentage (%). A t-test was used for continuous variables, and the chi-square test was used for categorical variables. p<0.05 was considered statistically significant. Continuous variables are summarized as mean±SD values.
Results
1. Enrollment
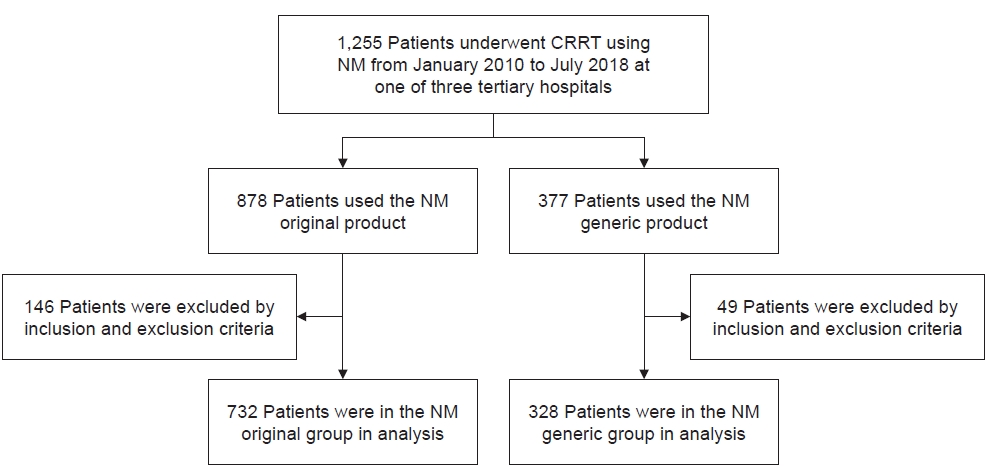
Between January 2010 and July 2018, a total of 1,255 patients received CRRT as defined above. After data collection, 146 patients were excluded from the original product group and 49 patients were excluded from the generic product group. In the final analysis, we included 732 patients in the original product group and 328 patients in the generic product group (Fig. 1).
2. Patient characteristics
The age of patients was 64.1±14.9 years in the original product group compared to 62.5±13.6 years in the generic product group. The proportion of patients with chronic kidney disease was 31.1% in the original product group versus 35% in the generic product group. The most common cause of death was multiple organ failure, which affected 60.1% of patients in the original product group and 52.2% of patients in the generic product group. Acute Physiology and Chronic Health Evaluation III scores were 83.3±34.1 points in the original product group and 82.9±37.1 points in the generic product group. There was no significant difference between product groups according to sex, age, chronic kidney disease, death, or oliguria at baseline.
In terms of CRRT modality, blood flow was 120±25.3 mL/hr in the original product group and 125.0±22.1 mL/hr in the generic product group. The CRRT dose was 32.0±10.2 mL/kg/hr in the original group and 30.0±9.5 mL/kg/hr in the generic group (Table 1).
3. Etiology of AKI
The cause of AKI varied in this study, though the most common was ischemia or shock, affecting 40.1% of patients in the original product group and 51.2% of patients in the generic product group. Other reasons included sepsis, nephrotoxin, rhabdomyolysis, urinary tract obstruction, multiple myeloma, and tumor lysis syndrome. A multifactorial cause was found in 13.8% of cases in the original product group and 8.2% of cases in the generic product group. There was no meaningful difference between the two groups in terms of the etiology of acute renal failure according to anticoagulant used (Table 2).
4. Reasons for starting CRRT
CRRT was initiated due to oliguria or anuria in 45.6% of patients in the original product group and 41% of patients in the generic product group. Fluid overload occurred in 28.9% of patients in the original product group and 26% of patients in the generic product group. Other, less common reasons included high blood urea nitrogen/creatinine levels, metabolic acidosis, and hyperkalemia (Table 3).
5. Filter characteristics
The mean (SD) filter lifespan was 36.3±15.1 hours in the original product group compared to 22.2±16.2 hours in the generic product group, with no statistically significant difference. The mean number of filters per day was 0.9±0.6 in the original product group but 1.7±0.7 in the generic product group, also with no statistically significant difference. Finally, the mean TMP was 62.2±47.3 mmHg in the original product group versus 74.5±45.6 mmHg in the generic product group (Table 4).
Discussion
The results of this study showed no significant difference in filter lifetime of the original and generic versions of nafamostat mesylate. However, transmembrane potential (TMP), a parameter indicating intra-circuit obstruction as the pressure gradient on the two sides of the filter membrane, tended to be lower with the original version than with the generic version. An increase in TMP can be secondary to membrane clogging or some other form of clotting along the circuit [8,9]. Although there was no difference in filter lifetime between the formulations, use of the original version of nafamostat mesylate may be advantageous because it has a lower TMP than the generic version. The results of the present study showed no meaningful difference between the two groups in terms of patient characteristics, etiology of AKI, or reason for CRRT.
Nafamostat mesylate is a synthetic serine protease inhibitor that inhibits coagulation factors and platelet aggregation. Thus, nafamostat mesylate has been used as an anticoagulant in CRRT [6]. In Korea and Japan, when CRRT is performed in patients with a tendency to bleed significantly, anticoagulation is often performed using nafamostat mesylate. Therefore, several generic versions of nafamostat mesylate have been released, and research on each product has become necessary as the range of choices has increased. In this study, we compared the circuit patency and exchange rates of the original nafamostat mesylate and a generic version to conclude which is more efficient as an anticoagulant in CRRT.
Although there was no meaningful difference in filter life between the two groups in this study, other such research has been published. In one study, the continuous hemodiafiltration run time of the generic product group was significantly shorter than that of the original product group (retrospective, n=24: generic 22.8±12.8 vs. original 36.3±10.3, p<0.01; prospective, n=7: generic 17.4±10.1 vs. original 32.3±13.3, p<0.01). Niwa et al. [10] used high-speed liquid chromatography to analyze the subcomponents in the original and a generic version of nafamostat mesylate. While about 0.2% of the original product comprised additives, that composition of the generic product was significantly higher at 0.3% to 0.5%, and unknown additives not detected in the original product were detected in the generic version. Such difference in additives could affect the filter lifespan. Even in the present study, the difference in filter lifespan with the original versus generic version may be due to variation in additives [10].
In this study, the mean filter lifespan was shortened to <24 hours in the generic product group. In general, dialysis filters used in CRRT in intensive care unit patients should be used for ≥1 day [2,11]. However, the use of generic products that can cause intra-circuit obstruction within 24 hours should be decided in consideration of clinical situations.
Our study had several limitations. First, because this was a retrospective study, it is difficult to determine the cause-and-effect relationship. Second, although the difference was not statistically significant, it is necessary to analyze the mixtures of the original product and the generic product and conduct a prospective study in the future to confirm the filter lifespan in both groups. Third, it is necessary to assess platelet count, prothrombin time, activated partial thromboplastin time, and fibrinogen level in such patients. It is also necessary to check and compare the presence or absence of anticoagulation or antiplatelet drugs because it could affect circuit coagulation.
In conclusion, this retrospective study suggests no meaningful difference in filter lifespan between the original and generic versions of nafamostat mesylate. However, TMP was lower in the original product group than in the generic product group.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by a grant from SK Chemical and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2022R1C1C1010662).
Author contributions
Conceptualization: SH, YK, HSS. Data curation: SH, YK, NL, YNK, HSS. Formal analysis: SH, YK, HSS, YJ, HR. Funding acquisition: SH, YK, HSS. Investigation: SH, YK, HSS. Methodology: all authors. Project administration: SH, YK, HSS. Resources: SH, YK, HSS, YJ, HR. Software: all authors. Supervision: SH, YK,HSS, YJ, HR. Validation: all authors. Visualization: all authors. Writing - original draft: SH, YK,HSS. Writing - review & editing: all authors. Approval of final manuscript: all authors.