A case report of successfully treated metachronous gastrointestinal stromal tumor and colon cancer
Article information
Abstract
The diagnosis of gastrointestinal stromal tumor (GIST) has become relatively common in recent years, but little is known about its association with other malignancies. We present a rare case of successfully treated metachronous GIST and colon cancer with concurrent FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) chemotherapy and imatinib. A 63-year-old man presented with abdominal pain that had started 2 weeks ago, and endoscopic ultrasonography showed masses that were compatible with GIST on the duodenum. He underwent Whipple surgery. One year after the GIST diagnosis, two liver masses were found on abdominal computed tomography images taken for surveillance. A liver biopsy showed metastatic adenocarcinoma, not GIST. Colonoscopy was then performed to identify the primary site of the metastatic adenocarcinoma in the liver, and sigmoid colon cancer was found. He received 12 cycles of adjuvant FOLFOX concurrently with adjuvant imatinib. There were no serious adverse events of grade 3 or higher from either imatinib or chemotherapy. He has completed adjuvant imatinib and FOLFOX chemotherapy and there is no evidence of disease recurrence. When a synchronous or metachronous tumor is found in a GIST patient, the clinician should keep in mind the possibility of another primary tumor of different histopathology, as well as GIST recurrence.
Introduction
Gastrointestinal stromal tumor (GIST) is the most common gastrointestinal tract malignancy of mesenchymal origin. Most studies of GIST have reported that the worldwide incidence of GIST is approximately 10 to 15 cases per million people annually [1]. However, while it has been diagnosed relatively commonly in recent years, little is known about the association between GIST and other malignancies. Only a few cases have been reported of the synchronous or metachronous existence of GIST with other malignancies. And there are no treatment guidelines for both diseases simultaneously. Herein, we report a rare case of metachronous GIST and colon cancer in which adjuvant FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) chemotherapy was concurrently administered with imatinib.
Case
Ethical statements: This report was exempted from review by the Institutional Review Board (IRB) of Busan Paik Hospital (IRB No. 2021-11-074). Written informed consent was obtained from the patients to participate in the study.
A 63-year-old man presented with abdominal pain that started 2 weeks ago. The results of a physical examination were unremarkable. His vital signs were stable with blood pressure 110/70 mmHg, heart rate 77 beats/min, respiratory rate 16 breaths/min, and body temperature 36.3 °C. There were no laboratory abnormalities other than anemia with hemoglobin of 12.1 g/dL. There was a large extrinsic compression at the duodenum in endoscopy and a hypoechoic oval mass which is originated from the muscularis propria and is focally connected with serosa in endoscopic ultrasonography (Fig. 1). Endoscopic results were compatible with GIST. Computed tomography (CT) images showed marked circumferential wall thickening with dilatation of second and third portion of duodenum (Fig. 2). Neither metastasis nor other abnormal findings were observed on CT. He underwent Whipple surgery. The surgical specimen showed three masses and the largest was 9.0 cm. All were pathologically confirmed as GIST, and they showed 2 mitoses per 50 high-power fields. c-receptor tyrosine kinase (KIT) and cluster of differentiation 34 immunohistochemistry results were positive (Fig. 3). The patient started to take 400 mg of adjuvant imatinib mesylate daily for 3 years because he was a high-risk patient of GIST recurrence according to the revised National Institutes of Health risk criteria.
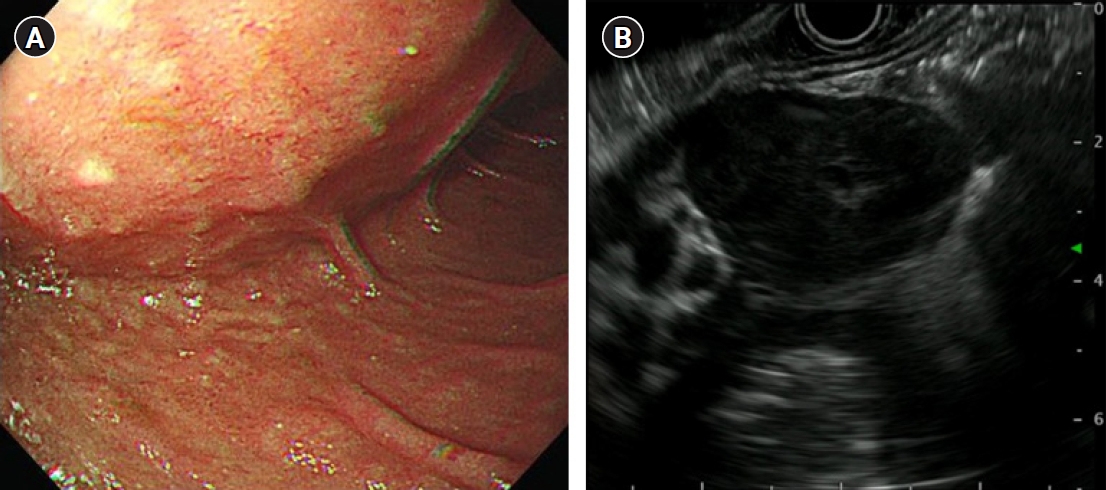
Endoscopic and endoscopic ultrasonographic findings of gastrointestinal stromal tumor. (A) Bulging at the duodenal second portion and (B) an inhomogeneous hypoechoic oval mass with regular margins and a largest diameter of 42 mm.
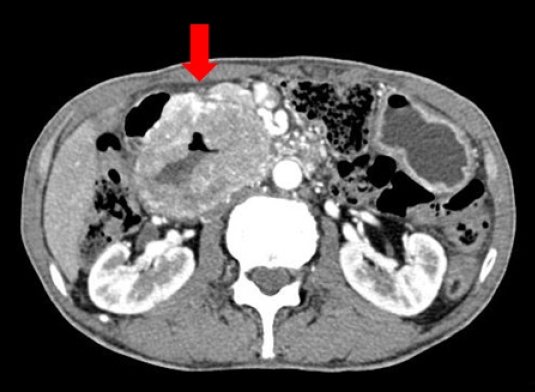
Computed tomographic findings of gastrointestinal stromal tumor. There was circumferential wall thickening with aneurysmal dilatation of the second and third portions of the duodenum (arrow). There was no bowel obstruction or organ invasion by the mass.

Gross and microscopic findings of the surgically resected gastrointestinal stromal tumor specimen. (A) Three exophytic oval masses (arrows). (B) A well-defined submucosal tumor (hematoxylin and eosin staining, ×40). The immunohistochemical staining results were positive for CD117 (C) and positive for CD34 (D) (×100). CD, cluster of differentiation.
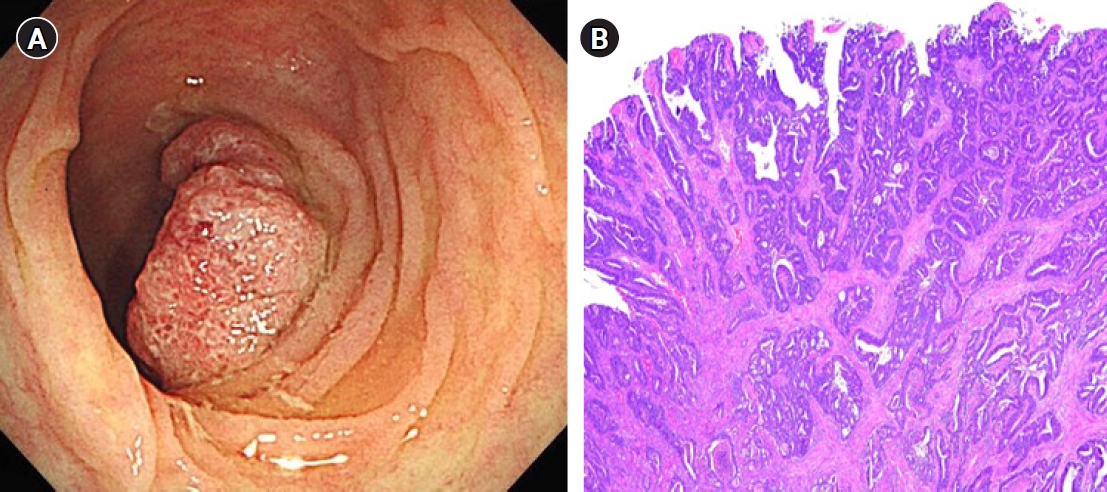
One year after the GIST diagnosis, two liver masses, 2.4 cm and 0.6 cm in size, were detected in an abdominal CT scan taken for surveillance (Fig. 4). Liver biopsy was performed to differentiate recurrence of GIST. However, the result of liver biopsy showed metastatic adenocarcinoma, not GIST. Colonoscopy was performed to identify the primary site of the metastatic adenocarcinoma of liver, and sigmoid colon cancer was found (Fig. 5). The patient then underwent low anterior resection and liver wedge resection. Pathologic results showed moderately differentiated adenocarcinoma with four out of 22 positive lymph nodes. The colon cancer was stage IVA according to the seventh edition of the American Joint Committee on Cancer Staging. He received 12 cycles of adjuvant FOLFOX chemotherapy (comprising an oxaliplatin 85 mg/m2; folinic acid 400 mg/m2; fluorouracil 400 mg/m2 followed by a continuous infusion of fluorouracil 2,400 mg/m2) concurrently with adjuvant imatinib. He experienced grade 2 neutropenia and peripheral neuropathy. There were no unexpected side effects from either imatinib or chemotherapy. He has completed adjuvant imatinib and FOLFOX chemotherapy and there is no evidence of disease recurrence for 4 years.
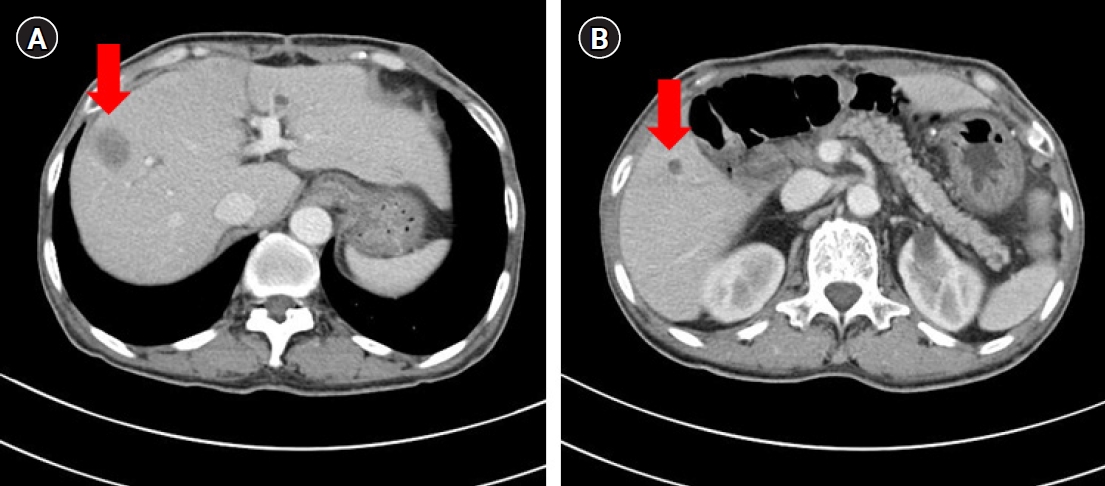
Computed tomographic findings of two liver metastases (arrow). (A) A 2.4-cm metastatic mass at liver segment 8. (B) An 0.6-cm metastatic nodule at liver segment 5.
Discussion
GIST is believed to arise from interstitial cells of Cajal present in the muscular layer of the gastrointestinal wall. It may occur anywhere in the digestive tract. The most common site of origin is the stomach (55.6%) followed by the small bowel (31.8%), colorectum (6.0%), and esophagus (0.7%) [1]. In addition to the gastrointestinal tract, it can also arise from the omentum, mesentery, and retroperitoneum. Although the incidence of GIST is increasing, little is known about the association between GIST and other malignancies [2]. Recently, many studies have reported data showing occurrence of other malignancies in patients with GIST [3-5]. The most commonly involved are stomach and colorectal malignancies [6-10]. These studies have reported an incidence of synchronous or metachronous gastrointestinal malignancy in patients with GIST up to about 30%. In addition to gastrointestinal malignancies, other tumors have been reported, such as renal cell carcinoma, uterine sarcoma, breast cancer, seminoma, and chronic myeloid leukemia [7,11]. Although there is no proven evidence of a common carcinogenic pathway between GIST and other malignancies, it is necessary to consider the possibility of the existence of another malignancy in patients with GIST. In our case, if liver metastasis on CT scan was mistakenly considered to be the GIST origin, and liver biopsy was not performed, we would have missed the diagnosis of colon cancer. Therefore, it is important to recognize that malignant lesions found during surveillance after GIST operation may not be GIST recurrence, but may be another malignant tumor with a different histopathology.
There are no prospective studies to investigate the optimal follow-up schedules and methods for resected GIST and the recommendations for surveillance vary in guidelines. The National Comprehensive Cancer Network guidelines recommend radiologic surveillance using abdominopelvic CT every 3 to 6 months for 3 to 5 years and then annually thereafter for completely resected primary GIST. The European Society for Medical Oncology guidelines also acknowledge that the optimal follow-up schedule is not known. Risk assessment based on the tumor size, tumor site, and mitotic count may be helpful in deciding the routine follow-up strategies [12]. In our case, the patient took a follow-up abdominopelvic CT scans every 3 months because he was at high risk of recurrence.
GIST was considered to be refractory to conventional chemotherapy or radiotherapy, and the prognosis was very poor until the 2000s. Over the last two decades, the molecular biology of GIST development has been revealed and its treatment has been markedly improved. Most GIST cases (approximately 80%–85%) are associated with mutations in the KIT gene. Platelet-derived growth factor receptor (PDGFR) mutation is an alternative oncogenic driver of GIST [13]. KIT or PDGFR mutations activate signaling pathways related to cell proliferation and apoptosis. Since mutations in these two genes are not present in all cases of GIST, it is believed that there may be mutations in other genes including rat sarcoma virus (RAS), succinate dehydrogenase (SDH), or rapidly accelerated fibrosarcoma (RAF). Imatinib, a tyrosine kinase inhibitor of KIT, has been approved for the treatment unresectable or metastatic GIST. It has also shown clinical benefit in the adjuvant setting. Imatinib is recommended for at least 3 years in patients with a high risk of recurrence after surgery [14,15].
In our case, the patient needed to receive adjuvant FOLFOX chemotherapy for colon cancer while he was taking adjuvant imatinib for GIST. We decided to administer both imatinib and FOLFOX chemotherapy because the drugs have different mechanisms of action against each cancer. But there is no proven data on the concurrent administration of imatinib and FOLFOX chemotherapy. We only found two case reports of imatinib and FOLFOX chemotherapy being concurrently administered in patients who were diagnosed with GIST and colon adenocarcinoma simultaneously [16]. In both cases, the patients were successfully administered both imatinib and FOLFOX chemotherapy without any unexpected side effects. Our patient also showed no other side effects except for grade 2 neutropenia and peripheral neuropathy. We suspect that the toxicity was tolerable because the duration of concurrent exposure to both drugs was short.
This is a rare case of metachronous colon cancer in a patient with GIST. Little is known about the association of these cancers. When a synchronous or metachronous tumor is found in the GIST patient, physicians should be alert to the possibility that tumors of different histological origin may coexist and careful diagnostic approach is required. Although further studies on efficacy and safety are needed, concurrent administration of FOLFOX chemotherapy and imatinib in patients with GIST and colon cancer can be considered as a treatment option very carefully.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: YJH, JYL. Data curation: YJH, JYL. Formal analysis: YJH. Investigation: YJH. Methodology: YJH. Project administration: JYL. Resources: JYL. Software: JYL. Supervision: JYL. Validation: JYL, YJH. Visualization: JYL, YJH. Writing - original draft: YJH. Writing - review & editing: JYL. Approval of final manuscript: all authors.