Traumatic neuroma of the right posterior hepatic duct with an anatomic variation masquerading as malignancy: a case report
Article information
Abstract
Traumatic neuroma (TN), also known as amputation neuroma, is a reactive hyperplasia of nerve fibers and connective tissue arising from Schwann cells after trauma or surgery. TN of the bile duct is usually asymptomatic, but rarely can lead to right upper quadrant pain, biliary obstruction, and acute cholangitis. It is very difficult to discriminate TN from malignancy before surgery, although doing so could avoid an unnecessary radical resection of the lesion. In the course of surgery, TN can be caused by unintentional injury of a nerve fiber near the common bile duct (CBD) and heat damage to an artery, complete ligation of an artery, and excessive manipulation of the CBD. Therefore, to prevent TN after cholecystectomy, surgery should be performed carefully with appropriate consideration of anatomic variations, and a cystic duct should not be resected too close to the CBD. The possibility of TN should be considered if a patient who has undergone CBD resection with hepaticojejunostomy or cholecystectomy long ago experiences symptoms of jaundice, cholangitis, or obliteration of the CBD. In this report, we present a case of TN mimicking cholangiocarcinoma that emerged from a cystic duct stump after cholecystectomy.
Introduction
Traumatic neuroma (TN) or amputation neuroma is a tumor-like hyperplasia that develops after trauma, surgery, or ischemia. It can occur in any location with nerve fiber distribution and can impact every nerve fiber, thereby causing symptoms. Considering that abundant nerve fibers are distributed near the common bile duct (CBD), TN that arises from the bile duct is often related to cholecystectomy [1]. In addition, TN is very difficult to differentiate from malignancy preoperatively; distinguishing them is important to avoid unnecessary radical resection of the lesion. In this report, we present a case of TN mimicking cholangiocarcinoma that emerged from a cystic duct stump after cholecystectomy.
Case
Ethical statements: This study was approved by the Institutional Review Board of the Pusan National University Yangsan Hospital (IRB No. 2023-0013). The need for written informed consent from the participants was waived because of the retrospective nature of this study.
A 65-year-old male visited the emergency room with abdominal pain on the right upper quadrant. His past medical history included cholecystectomy for cholecystitis 39 years ago. His physical examination results were as follows: blood pressure, 110/70 mmHg; pulse rate, 78 beats/min; respiratory rate, 20 breaths/min; and body temperature, 36.6 °C. There was no tenderness on the right upper quadrant abdomen and Murphy sign was negative. His laboratory tests included the following: white blood cells, 7,530/μL; hemoglobin, 11.7 g/dL; platelet, 158,000/μL; total bilirubin, 0.5 mg/dL; aspartate aminotransferase, 346 IU/L; alanine aminotransferase, 255 IU/L; alkaline phosphatase, 298 IU/L; gamma-glutamyl transferase, 604 IU/L; amylase, 40 IU/L; lipase, 7 U/L; C-reactive protein, 14.41 mg/dL; carcinoembryonic antigen (CEA), 4.44 ng/mL; and carbohydrate antigen 19-9, 25.3 U/mL. Viral markers for hepatitis A, B, and C were all negative, and blood culture showed no bacterial growth. Abdominal computed tomography revealed an 8 mm polypoid lesion at the right posterior hepatic duct (RPHD); intraductal papillary neoplasm of the bile duct was suspected (Fig. 1). On suspicion of cholangitis, we started empirical antibiotic therapy; subsequently, the symptoms and laboratory findings improved. In magnetic resonance cholangiopancreatography (MRCP), a triangular-shaped polypoid lesion at the RPHD was detected, suggesting early cholangiocarcinoma (Fig. 2). MRCP also revealed an anomalous variation of the RPHD directly draining into the common hepatic duct (Fig. 2). Positron emission tomography showed no evidence of a hypermetabolic lesion. Therefore, we performed endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound for the differential diagnosis of the RPHD lesion. ERCP revealed a focal filling defect with a smooth margin of the RPHD. In endoscopic ultrasound, a 7.8 mm hypoechoic lesion at the RPHD was noted, but infiltration into the hepatic duct wall was not clearly seen (Fig. 3). We decided a short-term follow-up because we could not rule out benign diseases such as fibrous change of the RPHD resulting from the previous cholecystectomy. One and a half month later, abdominal computed tomography identified an interval increase in size of the polypoid lesion at the RPHD, with strong contrast enhancement (Fig. 4). Laboratory test included the following: aspartate aminotransferase, 17 IU/L; alanine aminotransferase, 29 IU/L; alkaline phosphatase, 62 IU/L; C-reactive protein, 0.54 mg/dL; CEA, 7.78 ng/mL; and carbohydrate antigen 19-9, 16.6 U/mL, and only CEA was increased. In ERCP, the filling defect of the RPHD became more prominent than that in the previous ERCP (Fig. 5). Hence, biopsy through ERCP was performed, but the pathologic result showed only a few benign biliary epithelial strips. Eventually, we decided to perform a surgery, considering that cholangiocarcinoma could not be excluded because of the progress of the lesion and the repeated right upper quadrant pain during the follow-up period. Intraoperatively, the palpable mass was found at the RPHD. The common hepatic duct and CBD remained patent. Therefore, we performed right posterior sectionectomy of the liver with a negative surgical margin of the RPHD (Fig. 6). The patient’s postoperative course was uneventful, and he was discharged without any complication. Surgical pathology revealed TN, with no evidence of malignancy. Microscopically, an oval-shaped nodule was observed under the biliary epithelium. The nodule consisted of haphazardly arranged spindle cells with no nuclear atypia. Immunohistochemically, the spindle cells positively reacted to S100 protein (Fig. 6).

An 8-mm polypoid lesion at the right posterior hepatic duct; (arrow) an intraductal papillary neoplasm of the bile duct was suspected. (A) Axial view. (B) Coronal view.
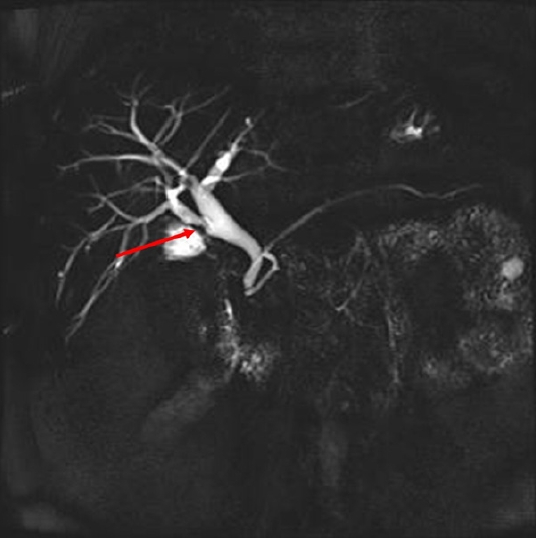
A triangular-shaped polypoid lesion at the right posterior hepatic duct was detected (arrow), suggesting early cholangiocarcinoma.

A focal filling defect with a smooth margin at the right posterior hepatic duct (arrow). (A) Endoscopic retrograde cholangiopancreatography and (B) endoscopic ultrasonography.

An interval increase in the size of the polypoid lesion at the right posterior hepatic duct, with strong contrast enhancement (arrow). (A) Axial view. (B) Coronal view.
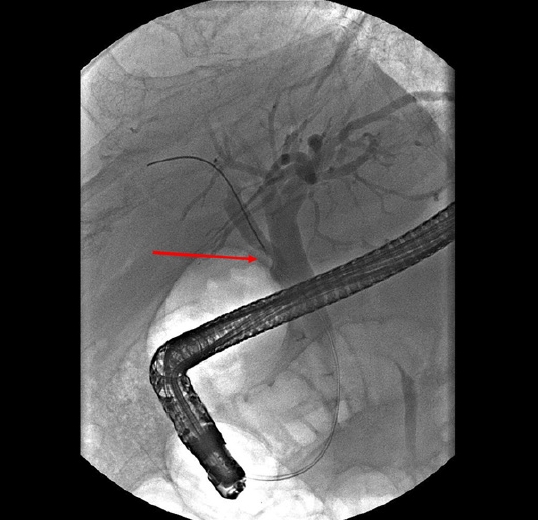
The filling defect of the right posterior hepatic duct became more prominent than that in the previous endoscopic retrograde cholangiopancreatography (arrow).

(A) Right posterior sectionectomy of the liver with a negative surgical margin of the right posterior hepatic duct (RPHD). RPHA, right posterior hepatic artery; RPPV; right posterior portal vein. Oval-shaped nodule, consisting of haphazardly arranged spindle cells with no nuclear atypia. Immunohistochemically, the spindle cells positively reacted to S100 protein (B: hematoxylin and eosin stain, ×40, C: ×40).
Discussion
TN, also known as amputation neuroma, is a reactive hyperplasia of the nerve fiber and connective tissue arising from Schwann cells after a trauma or surgery. Especially, TN of the bile duct is usually asymptomatic; in rare cases, it can lead to right upper quadrant pain, biliary obstruction, and acute cholangitis. TN most commonly occurs in the cystic duct stump after a laparoscopic or open cholecystectomy, with a range of intervals from several months to 45 years [2]. Our patient underwent open cholecystectomy 39 years ago and had atypical variations of the RPHD draining into the common hepatic duct directly. Therefore, the cystic duct might directly emerge from RPHD, and its stump might develop TN, resulting in recurrent right upper quadrant pain.
Discriminating TN from malignancy preoperatively is crucial to avoid unnecessary radical resection of the lesion, but it is extremely difficult. In this case, intraductal growing-type neuroma was very difficult to differentiate from malignancy by imaging study, making the pathological diagnosis even more challenging. Despite specimen harvest, the TN is still difficult to be confirmed as a nonmalignant lesion. Thus, great efforts are needed to avoid unnecessary radical resection of the lesion. For instance, an intraductal papillary lesion that develops in a patient who underwent CBD resection with choledochojejunostomy should be distinguished from cholangiocarcinoma.
Our patient only underwent right posterior sectionectomy without hepaticojejunostomy (HJ). Considering the severe adhesion caused by the previous surgery and the fibrotic change resulting from chronic inflammation, HJ was not performed to avoid postoperative complications. Additionally, an anatomic variation of the RPHD draining directly into the CBD was noted; thus, independent right posterior sectionectomy was possible. However, if no anatomic variation of the RPHD and development of TN occurred in the CBD, Roux-en-Y choledochojejunostomy would be preferable.
Nerve fibers are abundantly distributed around the CBD but are most intensively distributed around Calot triangle. TN can be developed from this area. Trauma, surgery, ischemia, or bleeding can cause proliferation of benign connective tissue from an injured nerve [3-5]. In the course of surgery, an unintentional injury of the nerve fibers near the CBD, as well as heat-induced arterial damage, complete arterial ligation, and excessive CBD manipulation, can cause TN. Hence, to prevent TN after cholecystectomy, surgeons should operate carefully while considering the anatomic variation and should not resect the cystic duct too close to the CBD.
In conclusion, TN is possible if a patient with a history of CBD resection with HJ or cholecystectomy manifests jaundice, cholangitis, or CBD obliteration. So, TN and malignancy need to be distinguished in clinical practice to avoid unnecessary radical resection of the lesion.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: KY. Data curation: JRS, TBL. Formal analysis: KY, JRS. Investigation: KY, JRS. Methodology: KY, JRS, TBL. Project administration: JHR. Resources: KY. Supervision: KY, BHC, JHR. Validation: none. Visualization: JRS, TBL, JHL. Writing - original draft: KY, JRS. Writing - review & editing: JRS. Approval of final manuscript: all authors.
