Mucinous carcinoma of the breast: distinctive histopathologic and genetic characteristics
Article information
Abstract
Mucinous carcinoma is a rare histologic type of breast cancer that, when classified with favorable histology, can be treated with different therapeutic options. This study reviews the histologic findings of mucinous carcinoma that support or exclude favorable histology and emphasizes the necessity of an appropriate gross examination with radiologic findings for an accurate diagnosis. In addition, unusual findings such as micropapillary arrangements and lobular differentiation in mucinous carcinoma and their implications for prognosis and treatment are reviewed. Mucinous carcinoma involves upregulation of MUC2, a mucus-associated gene common in mucinous carcinoma of the breast as well as various other organs. In mucinous carcinoma, the fraction of genome altered and tumor mutation burden are lower than those of invasive carcinoma of no special type, the most common histology of breast cancer. In addition, the genetic alterations found in mucinous carcinoma are diverse, unlike the pathognomonic genetic alterations observed in other histologic types of breast cancer. These genetic features support the importance of conventional microscopic evaluations for the pathologic differential diagnosis of mucinous carcinoma of the breast in routine practice. A variety of breast lesions, including mucinous cystadenocarcinoma and mucocele-like lesions, as well as mucinous carcinoma from other organs, can mimic mucinous carcinoma of the breast. In order to obtain an accurate pathologic diagnosis, careful evaluation of the overall histopathologic characteristics and ancillary testing are required to provide information on appropriate treatment and prognosis.
Introduction
Mucinous carcinoma of the breast (MCB) is a rare histologic type of invasive breast carcinoma, accounting for approximately 2% of breast carcinomas [1]. In terms of its low rates of local and distant recurrence and high rate of disease-free survival, it has been categorized as indolent breast cancer with favorable histology [2,3]. Therefore, accurate diagnosis of MCB is important because it can avoid improper application of endocrine therapy or chemotherapy, the mainstays of breast cancer, allowing for more appropriate treatment [4].
This article reviewed the histopathologic characteristics of MCB, including uncommon findings, with an emphasis on prognostic value. In addition, the histopathologic and genetic characteristics of MCB were reviewed to determine their significance in differential diagnosis of breast lesions.
Histopathologic characteristics of mucinous carcinoma
MCB is one of the favorable histologies of breast cancer that can be treated differently from general breast cancer. However, favorable histology is only for pure types of MCB [4].
As a general rule for histologic types of breast cancer, pure MCB is defined as an invasive carcinoma where more than 90% of the lesion is made up of mucinous components [5]. If mucinous components constitute between 10% and 90% of the overall lesion, the diagnosis is "mixed MCB with other non-mucinous histologic types"; if mucinous components make up less than 10% of the overall lesion, it is classified as a "non-mucinous histologic type." In the latter, the focal element of the MCB should be described following pathologic diagnosis. The assessment of the proportion of mucinous components is based on the overall area occupied by the cancer. Therefore, such a proportion can only be fully assessed from surgical specimens and not small biopsy tissue. In addition, when MCB is observed in surgical specimens, careful gross examination and tissue sampling are required to ensure identification of non-mucinous histologic types [3].
Mucinous components in the majority of MCBs produce glistening, gelatinous, or mucoid features on gross examination, allowing for a large number of MCBs to be well-demarcated from surrounding breast parenchyma [6]. However, Memis et al. [7] found an association between the type of border of MCBs on mammograms and the volume of mucinous components. They reported that all cases of pure MCB with mucinous components greater than 80% were well-defined, while all cases of mixed MCB were poorly defined or spiculated. However, pure MCBs with less than 80% mucinous components exhibit both types of borders. Similar results have been reported by other investigators [8,9]. Therefore, if gross examinations reveal a glistening, gelatinous, or mucoid breast mass suggestive of MCB, pathologists should locate and sample areas with poorly defined or spiculated borders during gross examination, based on a review of the radiological findings.
Magnetic resonance imaging (MRI) can provide additional clues for an appropriate gross examination based on contrasting findings of mucinous and non-mucinous components of the MCB. MRIs for pure MCBs present with hyperintense T2 signals and fat-saturated T2-weighted sequences with low signal intensity on diffusion-weighted imaging, corresponding to a high apparent diffusion coefficient [6]. These findings are the result of abundant water molecules that diffuse freely within the mucin pools that are characteristic of MCB. In contrast, non-mucinous components of mixed MCBs present with hypointense T2 signals and lower apparent diffusion coefficient [10]. Dynamic contrast-enhanced MRIs also exhibit useful and contrasting enhancement patterns [8]. In contrast to pure MCBs, which exhibit mild or strong, gradual enhancement in the early phase and strong and heterogeneous enhancement in the delayed phase, mixed MCBs are characterized by strong and heterogeneous enhancements in both early and delayed phases.
Even if the morphological characteristics of pure mucinous carcinoma are confirmed, a therapeutic approach to favorable histology can be considered only when typical ancillary test results such estrogen receptor (ER)-positive, progesterone receptor (PR)-positive, and human epidermal growth factor receptor 2 (HER2)-negative are confirmed [4]. A recent study using the Surveillance, Epidemiology, and End Results (SEER) database for 10,593 cases of MCB reported a high expression rate for hormone receptors (93.6% ER-positive, 84.6% PR-positive) and a low expression rate for HER2 (2.7% HER2-positive) [11]. When MCB is immunoprofiled as hormone receptor-negative or HER2-positive, it can be considered highly unusual or discordant, requiring confirmatory testing [12-14].
The prototypical histopathologic findings for MCB include small clusters of hypocellular tumor cells of low-to-intermediate nuclear grade floating in abundant extracellular mucin. However, MCBs can exhibit tumor cells of high nuclear grade, and it is unresolved if these can be classified as invasive carcinoma of no special type (NST) with mucin production [15]. However, the National Comprehensive Cancer Network (NCCN) recommends considering these cases as invasive breast carcinoma of NST in terms of therapeutic approach, even in MCB with a typical immunoprofile [4].
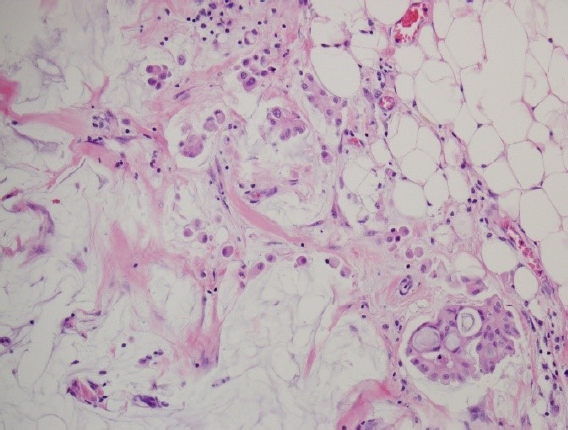
Capella et al. [16] reported that MCB is a heterogeneous disease consisting of types A and B, referred to as classical and endocrine variants, respectively. Unlike type A of prototypical histopathologic findings, type B exhibits distinct histopathologic features characterized by hypercellular tumor cells arranged in large clusters with a relatively small amount of extracellular mucin (Fig. 1). They described the detailed arrangement of type B as isolated or anastomosing clumps to sheet-like structures traversed by spaces, sometimes simulating cribriform structures. However, the latter was distinctly different from those of type A, usually consisting of aggregates of rings or annular growth patterns associated with trabeculae and ribbons. The amount of extracellular mucin in type B was less than that in type A. However, abundant extracellular mucin accounting for more than 50% of the total volume of the lesion, a consistent finding in type A, is also found in a minority of type B. Interestingly, abundant intracytoplasmic mucin was confined to some type B, while foamy cytoplasm was confined to some type A.
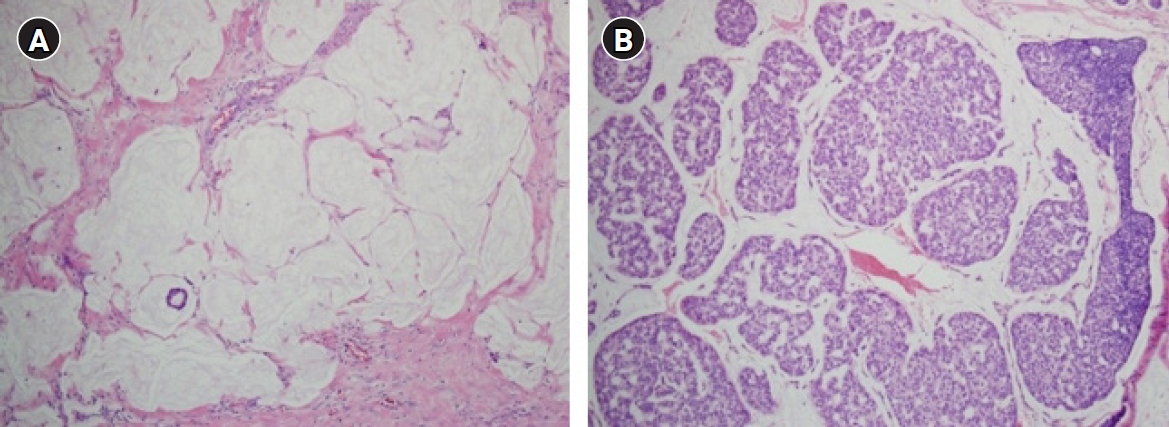
A comparison of mucinous carcinoma of the breasts (MCBs) with different cellularity. (A) Type A MCB is characterized by a small number of tumor cells forming small clusters and a background of abundant extracellular mucin (H&E, ×100). (B) Type B MCB is characterized by hypercellular tumor cells forming large clusters. The mucinous background between the clusters is relatively poor compared to that of type A (H&E, ×100).
They found neuroendocrine differentiation in type B, which was classified as an endocrine variant. Although the criteria for endocrine differentiation vary, it has been diagnosed in cases with an argyrophilia by histochemical reaction using Bodian and/or Grimelius stains, positive immunoreactions for neuron-specific enolase, chromogranin and/or synaptophysin, and dense endocrine granules on electron microscopy [16,17]. Tse et al. [17] acknowledged that it was difficult to evaluate such significance because of the good prognosis of MCB and death from other causes not related to breast cancer during the follow-up period due to the high proportions of elderly patients. In addition, they suggested that evaluation of independent prognostic factors such as nuclear grade and lymph node metastasis would be more feasible. From a practical point of view, neuroendocrine differentiation or cellularity of MCB is not a consideration for therapeutic strategies of MCB according to the recent NCCN guidelines and Korean clinical practice guidelines for breast cancer [4,18].
The histopathologic finding of MCB that has been proposed to be relevant to prognosis is the micropapillary arrangement of the tumor cells. MCBs with micropapillary features are characterized by tight morula-like and floret-like clusters common in micropapillary carcinomas, together with abundant extracellular mucinous background typical of MCB. These findings have been reported as invasive micropapillary mucinous carcinoma, mucinous micropapillary carcinoma, and micropapillary variant of mucinous breast carcinoma, and were described as “mucinous carcinoma with micropapillary features” in the World Health Organization (WHO) classification of breast tumors, fifth edition [5]. Although it remains controversial whether these combined histopathologic findings can be interpreted as genuine micropapillary variants of MCB or the mucinous counterpart of invasive micropapillary carcinomas, investigators have reported that more than half of the tumors exhibited lymphovascular invasion, resulting in increased regional lymph node metastasis and early recurrence in the skin and chest wall [19,20]. Furthermore, large retrospective studies have reported that invasive micropapillary carcinoma exhibits higher nuclear grade, HER2 overexpression or amplification, and Ki67 index compared to pure MCB [21,22].
Meanwhile, some investigators have reported that micropapillary arrangement does not contribute to poor prognosis [23-25]. In these studies, none of the cases studied presented with high nuclear grade or HER2-positive immunoreaction (3+). These conflicting results can be attributed to relatively small sample sizes and inconsistent diagnostic criteria. Considering the low prevalence of MCBs, the number with micropapillary features would be limited. Fortunately, there is excellent diagnostic agreement between pathologists assessing MCBs with micropapillary features [22]. The unique histopathologic findings of MCBs with micropapillary features are the reverse polarity of tumor cells and psammomatous microcalcifications [19]. Reverse polarity describes the inside-out growth pattern in micropapillary carcinomas of various organs, with its apical pole facing outward toward surrounding clear, empty stromal spaces, rather than facing the center of the tumor cell clusters (Fig. 2). It presents with scalloped or frayed edges and apical cytoplasmic snouts at the outer cell membrane, with surface glycoprotein (EMA and MUC1) at the stromal-facing peripheral membrane, and with cell adhesion proteins (E-cadherin and p120) at the basolateral membrane [26,27]. However, an immunoreaction suggestive of reverse polarity has been reported in pure MCBs and should be carefully interpreted in conjunction with the characteristic histopathologic findings [22,28].
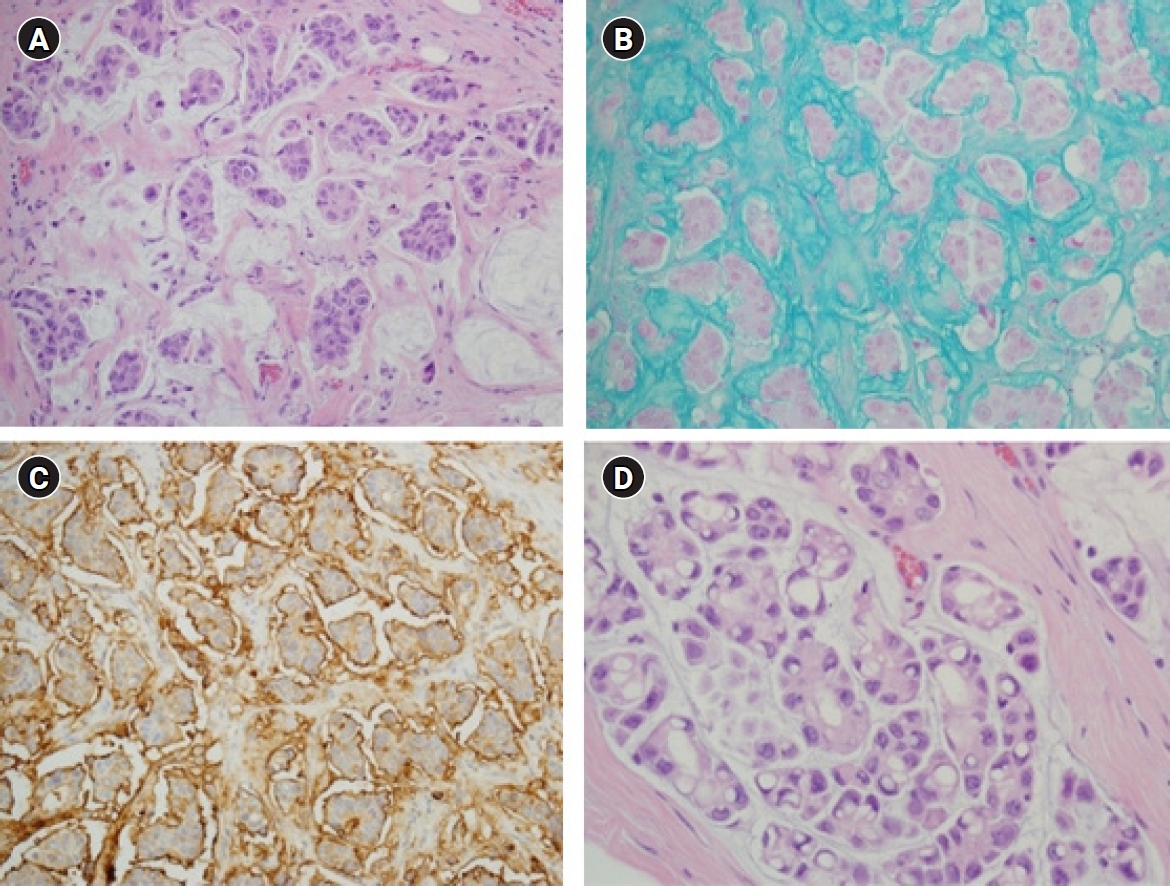
Mucinous carcinoma of the breasts (MCBs) with micropapillary features. (A) Tight morula-like clusters of intermediate to high-grade tumor cells are floating within the small spaces filled with extracellular mucin (H&E, ×200). (B) Mucus filling the spaces between the tumor cells and the surrounding stromal tissue is identified by Alcian blue staining (×200). (C) Immunohistochemical staining for epithelial membrane antigen is observed on the stromal-facing peripheral membrane of the tumor cells, clearly revealing the inside-out pattern (×200). (D) Abundant intracytoplasmic vacuoles are found in the focal area of MCB with micropapillary features (H&E, ×400).
Liu et al. [21] reported that invasive micropapillary MCB was found in approximately 25% of pure MCBs in a large retrospective cohort, amounting to 134 cases of invasive micropapillary MCB. Additionally, other investigators indicated that a micropapillary arrangement is not an uncommon finding in MCB [19,22,24]. However, well-defined diagnostic criteria such as a cutoff value for micropapillary elements, have not been established. Moreover, definitions of MCB with micropapillary features have not been consistent in previous investigations and were based on various morphologic and immunohistochemical findings [22]. Therefore, further investigations are needed to discriminate clinically aggressive breast cancers from pure MCBs in order to provide appropriate treatment and surveillance.
Another histopathologic finding associated with the prognosis of MCB is lobular differentiation (Fig. 3). Invasive lobular carcinoma with abundant extracellular mucin exhibits both extracellular and intracytoplasmic mucins, with tumor cells positive for MUC1, MUC2, MUC5AC, and MUC6 [29-31]. Abundant extracellular mucins are an unexpected finding in invasive lobular carcinomas that have exclusively intracytoplasmic mucins, but it remains unclear whether they should be classified as a type of either invasive lobular carcinoma or MCB. Burky et al. [32] summarized 31 published cases of invasive lobular carcinoma with abundant extracellular mucin and determined that it tended to have a higher nuclear grade (2 or 3) and more frequent HER2-positive status, suggesting an aggressiveness to these cases. However, the diagnostic criteria for invasive lobular carcinoma with abundant extracellular mucin have not yet been established, and various proportions of the mucin-producing lesions are reported to vary from 5% to 80% [31]. Distinguishing them from pure MCBs requires recognition of these unusual findings, and further studies are needed on the biomedical and therapeutic approaches for invasive lobular carcinoma with abundant extracellular mucin.
Genetic characteristics of mucinous carcinoma
Mucins can be broadly classified into secreted gel-forming mucins and membrane-bound mucins. The mucin gene expression signatures presented in MCB are upregulation of gel-forming mucins and downregulation of membrane-bound mucins [33]. Among the various mucin-related genes, MUC2, which encodes the epithelial glycoprotein MUC2, is the most commonly upregulated gene. The expression of MUC2 in MCB was negatively associated with the level of methylation of CpG mapped to MUC2, which has been suggested as a mechanism for the production of extracellular mucin [34]. Furthermore, Nguyen et al. [35] found a different methylation pattern in MCB, where MUC2 is hypomethylated at the promoter and the 5’-untranslated region and hypermethylated in more downstream exons. Upregulated MUC2 expression signatures have been reported in mucinous carcinoma of various other organs such as the colon, rectum, stomach, pancreas, gallbladder, and lung, as well as in MCB [33,36]. However, other molecular features such as increased microsatellite instability and increased BRAF, KRAS, and PIK3CA mutations that are commonly reported in mucinous carcinoma of non-mammary organs are not common in MCBs [36,37]. Differences in the molecular characteristics of MCB and mucinous carcinoma of various organs can explain the wide range of biological behaviors, suggesting that their pathogeneses are not identical.
Thennavan et al. [38] characterized the transcriptomic and genomic profiles of 24 pure MCB cases using a histological annotation dataset from The Cancer Genome Atlas Breast Cancer (TCGA-BRCA) project. They identified transcriptomic features of MCB including early estrogen response, late estrogen response, and protein secretion. Enrichment of estrogen-related genes overlaps similarly with that of other special histologic types of breast cancer such as cribriform, micropapillary carcinoma, and papillary carcinoma of luminal A/B subtypes. However, this contrasts with the immune-related and mitosis-related genes found in basal-like special histologic types such as metaplastic carcinoma and invasive carcinoma with medullary features. These results indicate the dominant biologic significance of genes shared by intrinsic subtypes. When analyzed using a transcriptome-based differentiation score, MCB expressed a mature luminal signature distinct from luminal progenitor and mammary stem cell signatures.
In comparison to ER-positive invasive carcinoma of NST, MCB has distinct genomic features. MCB is characterized by a low fraction of genome altered and presents as a diploid with neither chromosome 1q gain nor 16q loss, which are prevalent in ER-positive/HER2-negative, low-grade invasive carcinoma of NST [22,33,34,39]. In addition, a lower tumor mutation burden has been reported in MCB with less frequent TP53 or PIK3CA mutations compared to ER-positive/HER2-negative, low-grade invasive carcinoma of NST [34,39,40]. Interestingly, Pareja et al. [37] suggested that the mucinous and ductal components of mixed MCB were clonally related through either clonal selection or parallel evolution. However, the significance of the result was limited due to the small sample size (seven cases), and further investigations of clonality are needed to help elucidate the pathogenesis of mixed MCB.
As high-throughput sequencing becomes more accessible, some investigators have reported genomic features of MCB (Table 1). A low frequency of PIK3CA and TP53 mutations was common, as previously described. However, the genetic alterations frequently found in each study were diverse, and no pathognomonic mutations or genomic alterations have been identified in MCB. These discrepant findings might be influenced by the small sample size due to the relative rarity of MCB. However, they also might indicate the genomic diversity of MCB. The heterogeneity of the genomic drivers of MCB was also suggested in a genomic stratification study of 1,643 cases of breast cancer in the METABRIC (Molecular Taxonomy of Breast Cancer International Consortium) cohort [42]. That study found that the integrated clusters (IntClust) allocated by genomic and transcriptomic profiling and copy number analysis varied in MCB, in contrast to other histologic types of breast cancer with significant associations of specific IntClust.
Differential diagnosis of mucinous carcinoma
A mucinous background of breast lesions is a typical finding of MCB, but it can also be observed in a variety of other lesions of the breast. In terms of the absence of pathognomonic genomic findings in MCB, the pathological differential diagnosis of MCB in daily practice is within the scope of conventional microscopic evaluations.
Mucinous cystadenocarcinoma is exceptionally rare, with fewer than 30 cases in the literature, and was recently listed as a separate histologic type in the fifth edition of the WHO classification [43,44]. On macroscopic evaluation, most mucinous cystadenocarcinomas present as large cystic masses filled with gelatinous content, reminiscent of MCB [45]. However, this disease can be distinguished from MCB based on characteristic tumor cells lining multicystic spaces, creating stratification, tufting, or intracystic papillary structures that are not accompanied by myoepithelial cells. These tumor cells are tall columnar cells with abundant cytoplasmic mucin and basally located nuclei with various nuclear pleomorphisms. These tumor cells exhibit a triple-negative, basal-like phenotype that is ER-negative, PR-negative, HER2-negative, CK5/6-positive, and EGFR-positive, in contrast to the luminal phenotype of MCB [46]. Kim et al. [45] described tight clusters of tumor cells floating within abundant stromal mucin pools typical of mucinous cystadenocarcinoma. Therefore, tumor cells floating in mucin pools are not an absolute criterion for MCB, and comprehensive pathologic evaluation of the overall histologic findings in combination with immunohistochemical results is required. In addition, the characteristic tumor cells of mucinous cystadenocarcinoma are similar to those of mucinous cystadenocarcinoma of pancreatobiliary trees or ovaries [47]. To differentiate these diseases, it would be helpful to determine the histopathologic findings of concomitant ductal carcinoma in situ and establish a negative immunoreaction for CK20 and CDX2, in addition to obtaining clinical information on its systemic status [44].
Mucocele-like lesions are characterized by an acellular extracellular mucin pool that corresponds to extravasated mucin from dilated cysts. When mucocele-like lesions have epithelial strips floating in pools of mucin, they can mimic MCB. Since the epithelial strips involve epithelial lining dislodged from dilated cysts, determining the myoepithelial cells adhered to the epithelial cells might provide helpful diagnostic findings [15]. However, complicated pathologic findings such as various degrees of atypical epithelial proliferation, including atypical ductal hyperplasia, ductal carcinoma in situ, and invasive carcinoma, are not uncommon in mucocele-like lesions, hindering accurate diagnosis [48]. Despite reports that radiologic findings play a diagnostic role in epithelial proliferative lesions associated with mucocele-like lesions, the confirmatory diagnosis is based on histopathologic evaluation [49-52]. When an attenuated or proliferative epithelial lining appears along an extracellular mucin pool, the diagnosis favors mucocele-like lesions over MCB with prominent luminal locations of tumor cells floating within the mucin pool [52]. When the epithelial strips are small, deeper histologic sections and immunohistochemistry are helpful to differentiate mucocele-like lesions from epithelial proliferative lesions. Tan et al. [15] described that determining epithelial clusters in mucocele-like lesions can be difficult in some cases. Therefore, they suggested that it may be appropriate to acknowledge the uncertainty of these findings and the possibility of minute invasive foci.
Breast tissue is not an obligate origin of mucinous carcinoma, which can occur in various extramammary organs such as the gastrointestinal tract, pancreatobiliary trees, female genital tract, urinary bladder, and lung. A recent study based on data from TCGA analyzed 902 patients with mucinous carcinoma of colorectal, breast, pulmonary, gastric, endocervical, or pancreatic origin [33]. They found that mucinous carcinomas of independent tumor origin shared transcriptomic similarities such as upregulation of gel-forming MUC2, SEC16A, and CRACR2A and complementary genes involved in MUC2 packing, folding, and transport, suggesting pan-cancer biomarkers of mucinous histology. In addition, genomic similarities of fewer DNA copy number alterations in mucinous carcinoma have been reported across tumor origins. Therefore, histopathologic, transcriptomic, and genomic characteristics of mucinous carcinomas can be similar, irrespective of tumor origin.
Mucinous carcinoma that has metastasized from extramammary organs is rare in the breast, where primary breast lesions dominate [53]. Nevertheless, if a patient exhibits a clinical history of malignancy, especially with mucin-producing adenocarcinoma, pathological differential diagnosis should be considered. It is important to collect sufficient clinical information and perform a careful pathological evaluation for the differential diagnosis. When a site of primary origin is specified, it is helpful to correlate expected or known histopathologic and immunohistochemical findings of primary origin with those of breast lesions, allowing efficient and rapid diagnosis through relatively small numbers of immunohistochemical markers. On the other hand, if the breast lesion is suspicious of a metastatic lesion, but the primary origin has not been identified, an approach using multiple immunohistochemical markers that can cover a broad range of anatomic areas is required to identify a primary origin. Moreover, a single immunohistochemical marker is insufficient to define a specific primary origin. Therefore, a panel-based approach that integrates overall clinical, radiological, and microscopic findings is essential [54,55]. Table 2 summarizes the representative immunohistochemical markers for mucinous carcinomas from various primary origins [54,56-70]. However, the cases in the literature were selected according to various morphological criteria, and there were many differences in immunohistochemical details such as clones and interpreted criteria of immunohistochemical markers.
Conclusion
For an accurate diagnosis of MCB, which is a prerequisite for appropriate treatment, each pathological evaluation process such as macroscopic, microscopic, genetic, and ancillary examinations should be properly coordinated on basis of appropriate correlations with radiologic findings and medical history. In this study, the pathologic and genetic findings relevant to the prognosis of MCB were reviewed with a focus on diagnostic significance in daily practice.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
All the work was done by MJ.