Articles
- Page Path
- HOME > Kosin Med J > Volume 33(2); 2018 > Article
-
Case Report
Adrenal incidentaloma: a case of asymptomatic pheochromocytoma - Sang Yoong Park, Jong Cheol Rim, Hyun Chul Cho, Yoon Chan Lee, Jung A Kim, So Ron Choi
-
Kosin Medical Journal 2018;33(2):215-222.
DOI: https://doi.org/10.7180/kmj.2018.33.2.215
Published online: December 31, 2018
Department of Anesthesiology and Pain Medicine, Dong-A University Hospital, Busan, Korea.
- Corresponding Author: So Ron Choi, Department of Anesthesiology and Pain Medicine, Dong-A University Hospital, 26, Daesingongwon-ro, Seo-gu, Busan 49201, Korea. Tel: +82-51-240-5390, Fax: +82-51-247-7819, choisr@dau.ac.kr
• Received: July 29, 2016 • Revised: October 18, 2016 • Accepted: October 19, 2016
Copyright © 2018 Kosin University College of Medicine
Articles published in Kosin Medical Journal are open-access, distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,325 Views
- 1 Download
Abstract
- An incidentaloma is a tumor found incidentally without clinical symptoms or suspicion; the lesion may be adrenal, pituitary, or thyroidal. We report the case of an asymptomatic individual with preoperatively undiagnosed pheochromocytoma (size: 4.86 cm) that was revealed using elective nonadrenal surgical procedures. The patient demonstrated peri- and post-operative hypertensive crisis and tachycardia. Three days after the dramatic onset of symptoms, the patient expired due to pulmonary edema, multiple organ failure, and terminal sepsis, despite administration of extracorporeal membrane oxygenation-assisted cardiopulmonary resuscitation. A left medial kidney mass obtained at autopsy confirmed pheochromocytoma.
- A 178-cm, 80-kg, 42-year-old male patient with acute respiratory failure was brought to the emergency center on 100% oxygen Ambu bag ventilation with intubation. His chief complaints were rapidly progressive dyspnea, headache, and decreased urine output. His BP was 140/90 mmHg, and his heart rate (HR) was 130–140 beats/min. Electrocardiography (ECG) showed sinus tachycardia and T wave inversion with prolonged QT interval. Oxygen saturation, as measured using a pulse oximeter, was 70%. The patient was unconscious and both pupils were fully dilated and fixed. Respiratory crackles were heard over both lung fields. A chest radiograph showed widespread bilateral alveolar infiltrates suggestive of pulmonary edema. Initial laboratory findings included the following: WBC, 18380 cells/µl; sodium/potassium, 140/5.7 mmol/L; creatinine, 1.9 mg/dl; uric acid, 10.7 mg/dl; serum osmolality, 319 mOsm/kg; AST/ALT, 111/115 IU/L; amylase, 186 IU/L; lactate, 124.3 mg/dl; CK-MB, 61 U/L; creatine phosphokinase, 425 U/L; and troponin-I, 2.89 ng/ml. Blood clotting test with disseminated intravascular coagulation (DIC) panel results were as follows: APTT, 40.2 s; FDP, 64.9 µg/ml; and D-dimer, 31.79 ug/ml. Room-air arterial blood gas analysis results were as follows: pH, 6.98; PCO2, 52.8 mmHg; PO2, 92.6 mmHg; bicarbonate, 12.1 mmol/L; and base excess, −20.1 mmol/L. Urinalysis was unremarkable. Echocardiography revealed severe global hypokinesia of the left ventricle and apical ballooning with an estimated ejection fraction of 20% to 24%, which was consistent with stress-induced cardiomyopathy.
- The patient's breathing was managed by continuous mandatory ventilation with tidal volume of 500 ml, respiratory rate of 18 breaths/minute, and positive end-expiratory pressure 5 cm H2O in an oxygen-medical air mixture (FiO2 of 0.6). Initially, hyperkalemia was treated with intravenous (IV) sodium bicarbonate and calcium carbonate administered at different sites, while dextrose 50% (50 ml/bottle) + Humulin R (10 IU) infusion was maintained. Despite doubling the dose (20+40+80+160 mg) of furosemide up to 320 mg, the total urine output was 70 ml. Subsequently, an additional furosemide infusion of 30 mg/h was maintained. The patient was transferred to the intensive care unit (ICU) for severe metabolic acidosis, lactic acidosis, and deteriorating kidney function; continuous renal replacement therapy (CRRT) was administered by the end of hospital day 1.
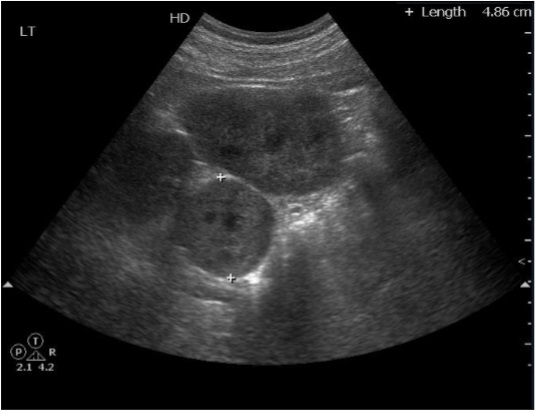
- The patient's medical records indicated that he had undergone elective arthroscopic bicep tendon repair surgery 4 hours previously. The patient had neither medical history nor symptoms relating to hypertensive problems. The preoperative electrocardiogram and chest X-ray were unremarkable and other laboratory findings were nonspecific. Upper abdomen ultrasonography showed a 4.86-cm round solid mass in the left abdomen, medial to the left kidney (Fig. 1). When the patient entered the operating room, the vital signs were as follows: BP of 150/80 mmHg, HR of 55 beats/min, normal sinus rhythm on ECG, and pulse oxygen saturation of 98%. At the beginning of the induction, the patient's hemodynamic changes progressively worsened; systolic and diastolic BP were 185 and 100 mmHg, respectively, with an HR of 100 beats/min. Anesthesia was induced with propofol 120 mg and succinylcholine 100 mg. Hemodynamic instability persisted after induction, and his BP remained fluctuated throughout the 90-minute procedure. To normalize the hemodynamic values, the patient was treated with lidocaine, 80 mg; fentanyl, 100 µg; hydralazine, 10 mg; esmolol, 30 mg (3 doses of 10 mg each); labetalol, 5 mg; and sodium nitroprusside infusion, 0.5 µg/kg/min. He was administered IV step-by-step and an increased sevoflurane concentration up to 5% vol. Despite adequate antihypertensive therapy during the entire surgical period, the BP and HR were not controlled (BP, 180–220/100– 140 mmHg; HR, 120 beats/min). Furthermore, ECG showed T wave inversion with prolonged QT intervals (Fig. 2). The patient was transferred to our emergency center less than 20 minutes after surgery and subsequently to the ICU for further management.
- On day 2, the BP had progressively decreased to 70/40 mmHg over a period of several hours. CRRT was provided, and dopamine and norepinephrine infusions were initiated with fluid administration. The patient's condition further deteriorated, despite efforts to maintain tissue oxygenation and adequate intravascular volume. The patient developed severe bradycardia with ST segment depression and was unresponsive to an atropine and epinephrine IV bolus. Following an undetectable mean arterial BP and an HR of 27 beats/min, cardiopulmonary resuscitation (CPR) was performed. An HR of 124 beats/min and mean arterial BP of 60 mmHg were restored. Within 8 minutes of initiating CPR, extracorporeal membrane oxygenation (ECMO) was initiated. The patient was connected to the crystalloid-primed ECMO circuit, and ECMO flow was started after approximately 1 hour of gradual BP drop. Vasopressin (0.03 U/min), dopamine (20 µg/kg/min), and norepinephrine (0.2 µg/kg/min) infusion sustained the cardiac index (CI) at 2.2–2.4 L/min/m2 and mean arterial BP at 70 mmHg. Despite maximal dose vasopressor and inotropic infusion, the CI and mean arterial BP gradually decreased to 0.3–0.5 L/min/m2 and 50 mmHg, respectively, over a few hours. The Levin tube and the endotracheal tube showed bleeding. The laboratory coagulation test with DIC panel suggested progressive DIC, i.e., PT, 38.5 s; peak international normalized ratio, 3.43; APTT, 76.9 s; fibrinogen, 136.2 mg/dl; antithrombin III, 46.2%; FDP, 132.9 µg/ml; and D-dimer, over 35 µg/ml. Cryoprecipitate, fresh frozen plasma, and packed red blood cells were transfused for 2 days as treatment for DIC and hypovolemic-induced hypotension.
- Three days after the dramatic onset of symptoms during surgery, the patient's vital signs abruptly fluctuated. His BP decreased to 52/35 mmHg, and his HR increased to 130 beats/min. One hour later, the patient suddenly developed an undetectable BP and HR. Complete asystolic cardiac arrest ensued and CPR was performed; however, the patient expired in 20 minutes despite 8 cycles of CPR. Post-mortem autopsy confirmed pheochromocytoma.
CASE
- The incidental finding of an adrenal mass on evaluation of a patient for a surgery may present a dilemma. For the present case, after incidentally finding the adrenal mass in the abdomen via upper abdomen ultrasonography, no evidence of pheochromocytoma could be detected. The patient did not present with symptoms of pheochromocytoma and the preoperative vital signs were within the normal range. Elective surgery under general anesthesia precipitated tachycardia and severe hypertensive crisis, associated with postoperative multiple organ failure (MOF) and pulmonary edema. For such conditions, development of a fast-track pathway to endocrine evaluation is valuable. Zeiger et al.3 recommended actively applying the AACE guidelines for diagnosing pheochromocytoma; however, these guidelines are not completely reliable for ruling out the diagnosis of pheochromocytoma. Grogan et al.7 reviewed 17 years of medical records for all patients undergoing adrenalectomy for incidentaloma by an endocrine surgery group. Of the 81 cases of incidentaloma found through imaging, 15 cases of pheochromocytoma were diagnosed through biochemical testing and one additional case was identified based on final histology after adrenalectomy. Menegaux et al.1 suggested that pheochromocytoma should be ruled out by biochemical testing of all adrenal incidentaloma patients, even those with normotensive preoperative status.
- In general, diagnosis is achieved through a combination of biochemical and imaging tests. Biochemical confirmation of plasma catecholamine concentrations and urine metanephrines are the standard screening tests for confirming the diagnosis of pheochromocytoma.8 In the present case, identification of adrenal incidentaloma using preoperative upper abdomen ultrasonography and evaluation of various symptoms in the patient when he presented at our emergency center led to the suspicion of undiagnosed pheochromocytoma. However, because of the many catecholamine stimulators in the drugs that are used to correct unstable vital signs, it was difficult to immediately conduct biochemical testing. Noninvasive localization by magnetic resonance imaging (MRI) is more sensitive than by an abdominal computed tomography (CT) scan, while MIBG scanning and positron emission tomography are other useful methods of diagnosis.9 Imaging tests were not conducted in our patient because of severe hemodynamic instability. Pheochromocytoma diagnosed postoperatively or by procedural intervention is correlated with serious perioperative risks, including a death rate of > 80%.6
- In this patient, the MOF involved pulmonary, cardiovascular, renal, coagulation system, and hepatic dysfunction. In a review of 31 female and 23 male autopsy-proven cases of pheochromocytoma at the Mayo Clinic over a 50-year period between 1928 and 1977, hypotensive or hypertensive crisis facilitated by surgery for unrelated conditions was a common cause of mortality. Intraoperative mortality was assigned variously to myocardial infarction, brain hemorrhage, ventricular arrhythmias, pulmonary edema, and wide fluctuations in BP.10 Siddik-Sayyid et al.11 reported a 43-year-old patient with an unusual presentation of pheochromocytoma, i.e., symptoms including MOF, disarrangements in BP consisting of hypertension or hypotension, and high fever. MOF may have resulted from high levels of plasma catecholamines, which can trigger immoderate vascular spasms, platelet aggregation, thrombosis, and volume contraction.6 Our patient developed severe tachycardia associated with hypertensive crisis, which was followed by acute pulmonary edema. Echocardiography showed global cardiac hypokinesia, which may have been secondary to a cardiomyopathy and is observed in conjunction with pheochromocytomas, as a result of high levels of plasma catecholamines extending to the myocardium.12 A previous report showed high mortality in patients with catecholamine-induced cardiomyopathy who presented with signs and symptoms of acute pulmonary edema, arrhythmia, nonspecific electrocardiographic changes, and congestive cardiac failure.
- We were required to decide between emergency resection and stabilization of vital signs with a pretreatment α-adrenergic blockade. For patients with diagnosed pheochromocytoma, many authors suggest emergency surgery for worsening conditions, despite maximal medical care. Bos et al.13 reported that emergency surgery for pheochromocytoma does not need to be strictly avoided and may be considered under life-threatening circumstances. However, May et al.14 reported that a patient who underwent emergency surgery for pheochromocytoma still exhibited complications from massive catecholamine excess, including severe tachycardia, hypotension or hypertension, renal failure, cardiac arrhythmia, shock, and adult respiratory distress syndrome. Postoperative hemodynamic instability, despite massive inotropic support and vasopressor therapy, and persistent renal failure, which necessitated CRRT and ECMO, resulted in patient mortality.
- Adrenal incidentaloma has a high possibility of excessive plasma catecholamine secretion by the adrenal mass during induction, and caution is required even in asymptomatic cases; light anesthesia with 120 mg of propofol per 80 kg weight and succinylcholine may stimulate more activity. Furthermore, confirmative diagnosis and further evaluation, such as biochemical testing, are required for analyzing adrenal masses.
- The patient should have been pretreated with α-blockers, notwithstanding the suspicion of pheochromocytoma. Early diagnosis and treatment were necessary for this patient. Once the diagnosis of pheochromocytoma was made, α-blockers could be administered, and once adequate alpha blockade was achieved, β-blocker administration could be initiated for preoperative management.
- In this case, ECMO was initiated and dopamine and norepinephrine infusions were administered for fulminant cardiopulmonary failure; however, the patient's condition continued to deteriorate. Fulminant cardiopulmonary failure unresponsive to conventional treatment can be fatal if mechanical cardiopulmonary life support (CLS) is not administered within adequate time. ECMO can successfully provide CLS and lead to recovery from critical status secondary to a pheochromocytoma. The decision to administer ECMO was delayed for the present case demonstrating an unsuccessful ECMO episode with CPR in a patient with a pheochromocytoma.
- In conclusion, this case report describes a patient with undiagnosed pheochromocytoma who underwent elective surgery under general anesthesia. The surgery precipitated severe tachycardia and a hypertensive crisis combined with pulmonary edema, followed by unresponsive postoperative MOF. Despite treatment comprising various drugs and performing surgeries, the patient died. A more thorough evaluation of adrenal incidentaloma via biochemical and imaging tests is required to prevent periand post-operative hypertensive crisis and tachycardia associated with undiagnosed pheochromocytoma.
DISCUSSION
- 1. Terzolo M, Bovio S, Pia A, Reimondo G, Angeli A. Management of adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab 2009;23:233–243.ArticlePubMed
- 2. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev 2004;25:309–340.ArticlePubMed
- 3. Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas: executive summary of recommendations. Endocr Pract 2009;15:450–453.ArticlePubMed
- 4. Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, et al. A survey on adrenal incidentaloma in Italy. Study group on adrenal tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab 2000;85:637–644.PubMed
- 5. Kasperlik-Zaluska AA, Roslonowska E, Slowinska-Srzednicka J, Otto M, Cichocki A, Cwikla J, et al. 1,111 patients with adrenal incidentalomas observed at a single endocrinological center: incidence of chromaffin tumors. Ann N Y Acad Sci 2006;1073:38–46.ArticlePubMed
- 6. O'Riordan JA. Pheochromocytomas and anesthesia. Int Anesthesiol Clin 1997;35:99–127.Article
- 7. Grogan RH, Mitmaker E, Vriens MR, Harari A, Gosnell JE, Shen WT, et al. Adrenal incidentaloma: does an adequate workup rule out surprises? Surgery 2010;148:392–397.ArticlePubMed
- 8. Veglio F, Morello F, Morra Di Cella S, Del Colle S, Rabbia F, Mulatero P. Recent advances in diagnosis and treatment of pheochromocytoma. Minerva Med 2003;94:267–271.PubMed
- 9. Lenz T, Gossmann J, Schulte KL, Salewski L, Geiger H. Diagnosis of pheochromocytoma. Clin Lab 2002;48:5–18.PubMed
- 10. Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma: Review of a 50-year autopsy series. Mayo Clin Proc 1981;56:354–360.ArticlePubMed
- 11. Siddik-Sayyid SM, Dabbous AS, Shaaban JA, Daaboul DG, Baraka AS. Catastrophic cardiac hypokinesis and multiple-organ failure after surgery in a patient with an undiagnosed pheochromocytoma: emergency excision of the tumor. J Cardiothorac Vasc Anesth 2007;21:863–866.ArticlePubMed
- 12. de Miguel V, Arias A, Paissan A, de Arenaza DP, Pietrani M, Jurado A, et al. Catecholamine-induced myocarditis in pheochromocytoma. Circulation 2014;129:1348–1349.ArticlePubMed
- 13. Bos JC, Toorians AW, van Mourik JC, van Schijndel RJ. Emergency resection of an extraadrenal phaeochromocytoma: wrong or right? A case report and a review of literature. Neth J Med 2003;61:258–265.PubMed
- 14. May EE, Beal AL, Beilman GJ. Traumatic hemorrhage of occult pheochromocytoma: a case report and review of the literature. Am Surg 2000;66:720–724.ArticlePubMedPDF
References
Figure & Data
References
Citations
Citations to this article as recorded by 


 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE


 PubReader
PubReader ePub Link
ePub Link Cite
Cite